Introduction
Patients may present to Emergency Departments (ED) in shock for various reasons. Shock states may be categorized as: cardiogenic, obstructive, distributive or hypovolemic. During initial resuscitation, an emergency medicine physician may also have to transiently deal with undifferentiated shock. While treatment of shock states is primarily aimed at reversing or resolving the cause of shock, emergency medicine physicians may require the use of vasopressors or inotropes to manage these patients. Vasopressors are agents that often act to increase mean arterial pressure by systemic vasoconstriction, while inotropes primarily act to increase cardiac output through a combination of inotropy, chronotropy and afterload reduction. Knowledge regarding which vasopressor or inotrope is most useful in which shock state is essential to the acute care physician, as is knowledge regarding appropriate venous access and potential side effects of the chosen agent. Vasopressor or inotrope use should not supplant rapid institution of definitive treatments for the identified cause of shock.
Current medical literature shows a paucity of evidence based guidelines to help the emergency medicine physician with vasopressor or inotrope use in shock states in the ED. The Critical Care Practice Committee of the Canadian Association of Emergency Physicians (C4) conducted an intensive literature search and guideline development process to help create an evidence based approach for use of these agents in the stabilization of shock.
Methods
Clinical need for a set of evidence based recommendations on vasopressor and inotrope use for shock was identified by members of C4 via informal clinical feedback, lecture and presentation evaluation and feedback and literature review. C4 itself is a heterogeneous group of emergency medicine physicians from across Canada, spanning urban, rural, tertiary and community EDs. Although vasopressor reviews are found in the medical literature, no evidence-based assessments directly relevant to the ED could be found.
Planning for the project occurred from June 2011 to December 2011. The AGREE II (Appraisal of Guidelines for Research and Evaluation II) instrument framework was used to guide project planning. 1 C4 members interested in the project formed the Vasopressor and Inotropes in Canadian Emergency department (VICE) subgroup. A set of seven PICO (Population, Intervention, Control, Outcome) clinical questions was established by February 2012. Two section authors were assigned for each question, and a non-voting project chair developed and coordinated the project. As members of the VICE group are spread across Canada, much of the project was conducted via email and teleconference. No industry funding was required to conduct the project.
A set of group derived, database appropriate keywords was created. The two authors of each section modified and expanded the search strategy to address their own section and performed their own literature review. Section authors were encouraged to search MEDLINE, EMbase, Cochrane Central, Register of Controlled Trials, Cochrane Database of Systematic reviews and the Cochrane Methodology Register and to enlist the help of a librarian or other expert in search strategy. A supplemental search with hand searches of the bibliographies of other literature reviews was also conducted where necessary. Each section author independently reviewed the literature list and identified appropriate studies. Articles identified by both section authors formed a focused literature list, and these articles were retrieved for full text review (Table 1).
Table 1 Literature search list

The VICE group utilized the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) system to assess and assign quality of evidence, and later to determine strength of recommendations.Reference Atkins, Eccles and Flottorp 2 – Reference Guyatt, Gutterman and Baumann 11 With use of their focused literature lists, section authors were asked to create recommendations for their assigned topic. Best evidence studies were short listed to identify quality of evidence (Table 1). As per GRADE, quality of evidence was rated A-D (A= high quality of evidence, B=moderate quality of evidence, C=low quality of evidence, D=very low quality of evidence). If best identified evidence consisted of randomized controlled trials, rating began as A, and was downgraded to B if poorly done. If best identified evidence was observational series, then grading began as C and could be upgraded to B if well done. Case series and expert opinions were relegated to D level of evidence (Figure A). 10 , Reference Guyatt, Gutterman and Baumann 11

Figure A Determination of quality of evidence using GRADE.
Once section recommendations were assigned appropriate grading of evidence, the VICE group utilized the Delphi consensus process to determine strength of recommendation. The Delphi technique is a widely used and accepted method of gathering data from participants within their domain of expertise, to bring structure and credibility to consensus-building efforts.Reference Graham, Regehr and Wright 12 , Reference Hsu and Sandford 13 GRADE asks that four main items be considered to determine strength of recommendation: balance between desirable and undesirable effects, quality of evidence, values and preferences, and costs (Figure B). 10 , Reference Guyatt, Gutterman and Baumann 11 VICE authors voted on each recommendation in order to assign a strength of recommendation rating of “strong” or “conditional” (weak) (Figure C). Seventy percent of votes for “strong” were required for this strength of recommendation to be assigned (Table 2).
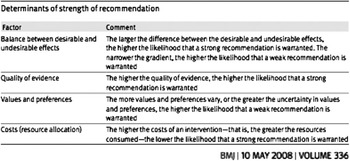
Figure B Determination of strength of recommendation in GRADE.

Figure C GRADE scoring.
Table 2 Delphi voting results to determine strength of recommendations
Results
Seven clinical questions were created to address vasopressor and inotrope use in Emergency Departments. Eighteen recommendations were created. Five recommendations were assigned as “strong” recommendations and thirteen recommendations were assigned as “conditional” (Table 3). Once in use, future external validation of the CPG is planned.
Table 3 Key recommendations summary

Question 1: For ED patients in shock, what are the side effects of vasopressors and inotropes?
Evidence based statements for side effects of vasopressors and inotropes were created and graded to assist in determining strength (strong versus conditional) of recommendations in other shock questions, but not to become recommendations in and of themselves.
Statement: Dopamine increases the risk of tachyarrhythmia compared to norepinephrine. (Grade A).
Rationale: A Cochrane review of vasopressor use analyzed six randomized controlled trials comparing dopamine to norepinephrine.Reference Havel, Arrich and Losert 14 Two studies documented a difference in arrhythmias including sinus tachycardia (25% versus 6%), atrial fibrillation (21% versus 11%), ventricular tachycardia (2.4 vs 1.0%), and ventricular fibrillation (1.2 vs 0.5%). In summary, dopamine produces more arrhythmias (RR 2.34; 95% CI (1.46, 3.78)). Arrhythmia consequences were not independently identified.
Two other systematic reviews analyzed those same six studies and also concluded significant decrease in arrhythmia with the use of norepinephrine compared to dopamine.Reference Vasu, Cavallazzi and Hirani 15 , Reference De Backer, Aldecoa and Nijmi 16
Statement: Dopamine use in septic shock increases mortality compared to norepinephrine (Grade B).
Rationale: The Havel Cochrane reviewReference Havel, Arrich and Losert 14 did not find a significant increase in mortality at 12 months with the use of dopamine compared with norepinephrine in all causes of shock combined. Another systematic reviewReference Vasu, Cavallazzi and Hirani 15 compared the same studies but with an outcome of mortality at 28 days. They found a decrease in mortality with the use of norepinephrine (RR: 0.91; 95% CI 0.83 to 0.99; p=.026) compared to dopamine. A third systematic reviewReference De Backer, Aldecoa and Nijmi 16 analyzed the previous studies once more, but only included patients in septic shock. One of the included studies had included a mixed population of shock, of which the subgroup of septic shock (1044 of 1659 patients) was extrapolated.Reference De Backer, Biston and Devriendt 17 This systematic review found that dopamine increased mortality at 28 days (RR 1.12; 95% CI 1.01–1.20; p=.035). It also assessed six observational studies in septic shock and dopamine again increased mortality at 28 days (RR, 1.23; 95% CI 1.05–1.43; p<.01).
Statement: Vasopressin as a first line vasopressor may be associated with cellular ischemia and skin necrosis, particularly when combined with sustained moderate to high dose infusions of norepinephrine. (Grade C).
Rationale: One study in septic shockReference Obritsch, Jung and Fish 18 demonstrated ischemia of the mesenteric mucosa, skin, and myocardium with vasopressin use. Elevated hepatic transaminase and bilirubin concentrations, hyponatremia and thrombocytopenia were also reported. A retrospective review of all patients who received vasopressin at a tertiary care adult regional burn centre demonstrated that vasopressin in combination with high dose norepinephrine is associated with intestinal necrosis, peripheral ischemia, skin graft failure, and donor site conversion.Reference Cartotto, McGibney and Smith 19 Limiting the dosage to 0.03 U/minute or less may minimize the development of these adverse effects.
Statement: Epinephrine increases metabolic abnormalities compared to norepinephrine. (Grade A).
Rationale: A prospective, double-blind, randomized-controlled trial of norepinephrine versus epinephrine in achieving mean arterial pressure in ICU patientsReference Myburgh, Higgins and Jovanovska 20 found that epinephrine was associated with the development of significant but transient metabolic effects (tachycardia, lactic acidosis, insulin requirements) that prompted the withdrawal of 18/139 (12.9%) patients from the study by attending clinicians. There was no difference in 28 and 90-day mortality.
Statement: Epinephrine increases metabolic abnormalities compared to norepinephrine-dobutamine in cardiogenic shock without acute cardiac ischemia. (Grade B).
Rationale: A small, open, randomized interventional studyReference Levy, Perez and Perny 21 evaluated two different therapies in acute heart failure without evidence of acute cardiac ischemia (epinephrine alone versus norepinephrine and dobutamine). Treatment with epinephrine is associated with a transient lactic acidosis, tachycardia, higher incident of arrhythmia, and inadequate gastric mucosa perfusion. No difference in mortality was observed.
Question 2: Which vasopressors and inotropes should be used in the treatment of ED patients with cardiogenic shock?
Recommendation: Cardiogenic shock patients in the ED should receive norepinephrine as the first-line vasopressor. (Strong)
Rationale: A large, high-quality, multicenter, randomized trial compared norepinephrine to dopamine as first-line vasopressor therapy for patients presenting in shock. While no difference in mortality was found between groups overall, the planned subgroup analysis of patients presenting in cardiogenic shock demonstrated that use of dopamine was associated with a significantly higher 28-day mortality.Reference De Backer, Biston and Devriendt 17 There was also a higher incidence of arrhythmia in the dopamine group overall. However, a recent Cochrane review of vasopressors for hypotensive shockReference Havel, Arrich and Losert 14 suggests that, because randomization in the DeBackerReference De Backer, Biston and Devriendt 17 study was not stratified by shock type, there is a possibility that the mortality difference in the cardiogenic shock subgroup could be due to chance. Yet, other evidence does exist to support the notion that dopamine may be a sub-optimal choice of vasopressor in cardiogenic shock. An analysis of a large, multi-center prospective observational cohort study,Reference Sakr, Reinhart and Vincent 22 suggests that dopamine use may be associated with a higher mortality in shock of all etiologies. This further supports the evidence in favour of norepinephrine.
Recommendation: Cardiogenic shock patients in the ED should receive dobutamine if an inotrope is deemed necessary. (Conditional)
Rationale: One small, open-label randomized trial of 30 patientsReference Levy, Perez and Perny 21 compared a combination therapy of titrated norepinephrine and fixed-dose dobutamine versus monotherapy of titrated epinephrine in patients with cardiogenic shock without evidence of acute cardiac ischemia. This trial demonstrated equal efficacy on global hemodynamics between the two regimens, but patients treated with epinephrine had higher lactate levels, higher heart rates, higher incidence of arrhythmias, and evidence of inadequate gastric mucosa perfusion, suggesting that the combination therapy might be preferable. Patients with active ischemia were excluded, and this group is responsible for the vast majority of cardiogenic shock encountered in the ED. The DeBacker trial,Reference De Backer, Biston and Devriendt 17 and an analysis from a large multi-center prospective observational cohort studyReference Sakr, Reinhart and Vincent 22 both provide evidence that dopamine use may be associated with higher mortality in shock of all etiologies, hence its use as first-line inotropic therapy is not recommended. The quality of the literature that directly compares dopamine with dobutamine is not particularly strong, and is largely performed in the context of severe heart failure patients, not specifically in cardiogenic shock. One small randomized crossover study of 13 patients compared hemodynamic variables in patients with acute cardiogenic circulatory collapse treated with dopamine vs dobutamine as single agent therapy,Reference Francis, Sharma and Hodges 23 and found that dobutamine improved stroke index and cardiac index significantly more than did dopamine, while dopamine increased LV filling pressure more than dobutamine.
Question 3: Which vasopressors and inotropes should be used in the treatment of ED patients with hypovolemic shock?
Recommendation: Routine vasopressor use in hypovolemic shock is not recommended. (Conditional)
Rationale: The use of any vasopressor in the treatment of hemorrhagic shock in humans has been evaluated thus far in scattered case reports, four medical record reviews, one prospective cohort study, and one RCT.Reference Cohn, McCarthy and Stewart 24 – Reference Sperry, Minei and Frankel 31 Two negative studiesReference Collier, Dossett and Mann 25 , Reference Sperry, Minei and Frankel 31 have shown an association with increased mortality in patients who received vasopressors, but the remaining studies show positive outcomes with respect to survival. Most studies involved the use of vasopressinReference Cohn, McCarthy and Stewart 24 , Reference Collier, Dossett and Mann 25 , Reference Haas, Voelckel and Wiedermann 27 , Reference Sharma and Setlur 29 , Reference Shelly, Greatorex and Calne 30 (see next section). Further study into the indications for vasopressor use in hemorrhagic or hypovolemic shock, and the preferred agent, are required.
Recommendation: Vasopressin may be indicated in hemorrhagic or hypovolemic shock if a vasopressor is deemed necessary. (Conditional)
Rationale: Promising studies of hemorrhagic shock in animal models have shown vasopressin improves organ perfusion and survival. In humans, there are three casesReference Haas, Voelckel and Wiedermann 27 , Reference Sharma and Setlur 29 of vasopressin use leading to return of spontaneous circulation or resolution of shock in patients with hemorrhagic shock unresponsive to fluids or catecholamines. Vasopressin has also been studied in a chart review and a weak RCT showing some benefit.Reference Cohn, McCarthy and Stewart 24 , Reference Shelly, Greatorex and Calne 30 However, Collier et alReference Collier, Dossett and Mann 25 showed that the use of vasopressin was associated with increased mortality in their retrospective cohort analysis. A multi-centre RCT is currently in progress to assess the rate of admission to hospital, fluid requirements, hemodynamic variables, and rate of discharge in a population of traumatic refractory hemorrhagic shock patients treated with vasopressin or placebo.Reference Wenzel 32
Question 4: Which vasopressors and inotropes should be used in ED patients with obstructive shock?
Recommendation: In obstructive shock not responding to indicated treatment, a systemically active vasopressor should be instituted. (Conditional)
Rationale: Evidence to elucidate vasopressor or inotrope use in obstructive shock is limited to case reports, case series and chart reviews. Case reports and case series related to massive pulmonary embolism have utilized various agents such as dopamine, norepinephrine, epinephrine, levosimendan and dobutamine, without the ability to make strong suggestions for care.Reference Boulain, Lanotte and Legras 33 – Reference Jardin, Genevray, Brun-Ney and Margairaz 35 The largest study is an observational review of 87 patients with pulmonary embolism in whom the use of norepinephrine or epinephrine was associated with worse ICU outcome, but no alternatives to treatment of the obstructive shock are offered.Reference Bahloul, Chaari and Kallel 36 Studies addressing cardiac tamponade are also not able to offer strong treatment suggestions.Reference Hata, Sezai and Iida 37 , Reference Kim, Siouffi and Silberstein 38 Physiologically it is reasonable to use vasopressors temporarily for the obstructive shock patient to preserve cerebral and cardiac perfusion until definitive treatment is instituted. Definitive treatment should be implemented emergently for obstructive shock patients.
Recommendation: For patients with known or suspected hypertrophic obstructive cardiomyopathy (HOCM) or dynamic outflow obstruction, inotropic agents should be avoided. Judicious use of vasoconstrictive agents can be considered. (Conditional)
Rationale: Only a series of three case reports could be found to specifically address this issue. One case reported decompensation of a patient intraoperatively after ephedrine administration,Reference Schmitto, Hein and Brauer 39 another case described cardiovascular collapse after a dopamine/norepinephrine combinationReference Auer, Berent and Weber 40 and one case reported successful use of phenylephrine during caesarean section.Reference Deimi, Hess and Bahlmann 41 Physiologically, it would seem reasonable to avoid the afterload reducing effects of inotropes in these fixed cardiac output states. If decompensation and mortality are imminent, it would seem physiologically sound to consider vasopressor support.
Question 5: Which vasopressors and inotropes should be used in ED patients with distributive shock?
Due to the numerous conditions that can cause distributive shock, independent sections and recommendations were created for septic shock, neurogenic shock, hepatic failure, anaphylactic shock, and adrenal insufficiency.
Recommendations: Norepinephrine is the first line vasopressor for use in septic shock. (Strong)
Rationale: Norepinephrine, epinephrine, phenylephrine, dopamine and vasopressin can all increase blood pressure in patients with septic shock.14 Older guidelines and reviews suggest that either norepinephrine or dopamine should be used as the first line vasopressor for septic shock.42 – 45 The 2012 Surviving Sepsis Guidelines suggest using norepinephrine as the first line agent for these patients.Reference Dellinger, Levy and Rhodes 46
Norepinephrine vs Dopamine
There are two recent large randomized controlled trials (RCTs) comparing mortality in patients with shock who were treated with dopamine or norepinephrine.Reference De Backer, Biston and Devriendt 17 , Reference Patel, Grahe and Sperry 47 One of the RCTs included only patients with septic shock, while the other included patients with all types of shock. The RCT with only septic shock patients demonstrated a mortality benefit in favor of norepinephrine.Reference Povoa, Carneiro and Ribeiro 48 In contrast, a recent large cohort study suggested higher mortality in patients treated with norepinephrine. This is contrasted by observational studies that demonstrated higher mortality in patients with septic shock who were treated with dopamine.Reference Sakr, Reinhart and Vincent 22 , Reference Boulain, Runge and Bercault 49 With respect to morbidity, the use of dopamine has been associated with an increased risk of dysrhythmias.Reference Havel, Arrich and Losert 14 , Reference De Backer, Aldecoa and Nijmi 16 There are two recent systematic reviewsReference Vasu, Cavallazzi and Hirani 15 , Reference Xu and Oziemski 50 and a meta-analysisReference De Backer, Aldecoa and Nijmi 16 that compare dopamine to norepinephrine. Two of the three concluded that norepinephrine confers a mortality benefit. All three demonstrated an increased risk of cardiac dysrhythmias when dopamine is used.
Epinephrine
The role of epinephrine in patients with septic shock is unclear. Although studies such as those by Seguin et alReference Seguin, Bellissant and Le Tulzo 51 and Levy et alReference Levy, Bollaert and Charpentier 52 have shown a difference in hemodynamic parameters and measures of tissue perfusion, two RCTs comparing norepinephrine (+ dobutamine where indicated) to epinephrine did not show a difference in mortality.Reference Myburgh, Higgins and Jovanovska 20 , Reference Annane, Vignon and Renault 53 The 2012 Surviving Sepsis Guidelines recommend the addition of epinephrine to norepinephrine “when [an] additional agent is needed to maintain adequate blood pressure”.Reference Dellinger, Levy and Rhodes 46 Mahmoud et alReference Mahmoud and Ammar 54 compared dobutamine to epinephrine for cardiovascular support in patients with septic shock who were already being treated with norepinephrine. This study did not show a difference in mortality between the groups. Serum lactate levels and pH were worse in the epinephrine group.
Phenylephrine
The pharmacologic properties of phenylephrine make it a less appealing choice during the setting of septic shock. There are trials comparing phenylephrine to norepinephrine in terms of hemodynamic and metabolic parameters showing equivalenceReference Jain and Singh 55 , Reference Morelli, Ertmer and Rehberg 56 or inferiorityReference Morelli, Lange and Ertmer 57 to norepinephrine. A 2011 review by Thiele et al discusses the evidence for the use of phenylephrine, including special situations where it might be the preferred agent.Reference Thiele, Nemergut and Lynch 58 Phenylephrine is not recommended for routine use in patients with septic shock.Reference Dellinger, Levy and Rhodes 46
Methylene Blue
Methylene blue can be administered as a bolus or an infusion. No studies have demonstrated a survival benefit with methylene blue. Methylene blue has been associated with positive physiologic effects such as increased MAP and SVR and reduced requirements for other vasopressors.Reference Andresen, Dougnac and Diaz 64 – Reference Kwok and Howes 67 Methylene blue can be considered for salvage therapy in septic shock refractory to fluids, catecholamines, and vasopressin.
Recommendation: Vasopressin should be considered in catecholamine refractory septic shock. (Conditional)
Rationale: The role of vasopressin in septic shock is unclear. Some patients with septic shock are vasopressin deficient but the clinical importance of this is unknown. When compared to norepinephrine as a first line agent for septic shock, vasopressin did not achieve MAP targets and rescue norepinephrine was required.Reference Lauzier, Levy and Lamarre 59 Vasopressin has been shown to reduce the dose of catecholamines required to achieve MAP targets in patients with septic shock.Reference Luckner, Dunser and Jochberger 60 The VASST study did not demonstrate a survival benefit when a vasopressin infusion was added to patients with septic shock who were already being treated with at least 5 mcg/min of norepinephrine.Reference Russell, Walley and Singer 61 Subgroup analysis of this study suggested that patients who were on lower doses of norepinephrine when vasopressin was initiated might have better survival. A later study by Oliveira et al (2011) demonstrated a reduction in 14 and 28 day mortality when patients were started on vasopressin in the setting of low dose norepinephrine (approx. 3.5–10 mcg/min).Reference Oliveira 62 In 2008, Lam and Bauer demonstrated similar results.Reference Lam and Bauer 63
Recommendation: Dobutamine should be used for septic shock with low cardiac output despite adequate volume resuscitation. (Strong)
Rationale: Cardiac dysfunction (septic cardiomyopathy) is common in septic patients. The presence of low cardiac index and the need for inotropes has been associated with increased 90-day mortality in patients with septic shock.Reference Wilkman, Kaukonen and Pettila 68 Earlier studies demonstrated that dobutamine improved cardiac index as well as creatinine clearance and gastric microcirculation.Reference Seguin, Bellissant and Le Tulzo 51 , Reference Vincent, Roman and Kahn 69 , Reference Hai-Bo, Yi and Shao-Xia 70 More recent studies on the use of dobutamine in septic shock also demonstrated an improvement in cardiovascular parameters, but only limited improvement in microcirculation.Reference De Backer, Creteur and Dubois 71 – Reference Hernandez, Regueira and Bruhn 73 Dobutamine was included in Rivers’ study of early goal directed therapy.Reference Rivers, Nguyen and Havstad 74 Dobutamine is recommended in the 2012 Surviving Sepsis Guidelines for patients who demonstrate low cardiac output despite volume resuscitation.Reference Dellinger, Levy and Rhodes 46
Recommendation: Vasopressor choice in neurogenic shock is not clear. The agent should be determined by patient characteristics and response to treatment. (Conditional)
Rationale: There are no studies comparing agents for the management of hypotension due to neurogenic shock. Several reviews suggest that, based on physiology, agents with beta agonist effects, such as dopamine, may be preferred over those with only alpha effects such as phenylephrine. We are not addressing the use of vasopressors to achieve supra-normal blood pressure following spinal cord injury in order to attempt to reduce the severity of injury. We have, however, included a review by Ploumis et al that looked at the ability of various agents to achieve supra-normal BP, and did not identify a difference between them.Reference Ploumis, Yadlapalli and Fehlings 75 If these agents all effectively achieve supra-normal BP, it seems reasonable to expect that they would also achieve a ‘normal’ BP target and thus would be effective for the management of hypotension due to neurogenic shock. Given the lack of evidence, the choice of agent used is determined by patient characteristics and response to treatment.
Recommendation: Norepinephrine is the first line agent for the management of distributive shock due to hepatic failure. (Conditional)
Rationale: There are no studies comparing vasopressor agents in patients with distributive shock due to hepatic failure. Recent reviews and guidelines suggest norepinephrine as a first line therapy based on a physiologic rationale.Reference Auzinger and Wendon 76 – Reference Stravitz, Kramer and Davern 83 The role of vasopressin in patients with hepatic failure is also unknown, but may be considered as a 2nd line agent for distributive shock due to liver failure that is not responsive to norepinephrine.Reference Lee, Larson and Stravitz 79 , Reference Olson, Wendon and Kramer 84 There is physiologic evidence to support the use of vasopressin to improve MAP in these patients.Reference Wagener, Kovalevskaya and Minhaz 85
Recommendation: Epinephrine infusion is the preferred agent for anaphylactic shock that does not respond to intramuscular or intravenous bolus epinephrine. (Strong)
Rationale: There are no randomized studies looking at the use of epinephrine for anaphylactic shock in humans. A recent Cochrane review on the use of epinephrine for the treatment of anaphylaxis did not find any studies suitable for analysis.Reference Sheikh, Shehata and Brown 86 A cohort study that analyzed the safety of an epinephrine infusion in patients with anaphylactic shock did not demonstrate any significant adverse effects.Reference Brown, Blackman and Stenlake 87 Studies in animals have demonstrated potential benefit with the use of phenylephrine, norepinephrine, vasopressin and methylene blue in animal models of anaphylactic shock.Reference Dewachter, Jouan-Hureaux and Lartaud 88 – Reference Zheng, Barthel and Chantal 90 There are multiple case reports describing the use of vasopressin, norepinephrine and methylene blue in patients with anaphylaxis refractory to epinephrine.Reference Hebard and Ball 91 – Reference Williams, Denault and Pellerin 101 Consensus guidelines recommend the use of an epinephrine infusion if a vasopressor infusion is required for patients with anaphylactic shock. 102 – Reference Soar, Perkins and Abbas 107
Recommendation: Vasopressor choice in distributive shock secondary to adrenal insufficiency not responding to steroid replacement is not clear. Patient response to chosen agents should guide therapy. (Conditional)
Rationale: There are no human trials comparing vasopressors in the setting of adrenal insufficiency. Steroid replacement needs to be instituted as soon as possible. Human case reports of adrenal insufficiency frequently describe reduced responsiveness to catecholamines. No recommendations for the use of a particular vasopressor can be made in these patients.Reference Matsumoto, Hagiwara and Kusaka 108 – Reference Serrano, Jimenez and Brouard 110 Several studies demonstrated vasopressin insufficiency in patients with septic shock and laboratory diagnosed adrenal insufficiency; however, there are no trials demonstrating that vasopressin provides a survival advantage in this group.Reference Lauzier, Levy and Lamarre 59 – Reference Lam and Bauer 63
Question 6: Which vasopressors and inotropes should be used in ED patients with undifferentiated shock?
Recommendation: In undifferentiated shock not responding to fluid resuscitation, norepinephrine should be the first-line vasopressor. (Strong)
Rationale: A large, multicenter, double-blinded randomized controlled trial showed equivalence between norepinephrine and dopamine in patients with shock; however, dopamine was associated with an increased rate of arrhythmias and a significantly increased rate of death in cardiogenic shock.Reference De Backer, Biston and Devriendt 17 Additionally, a large, multicenter, observational cohort study showed that dopamine was associated with increased mortality rates and that dopamine was an independent risk factor for ICU mortality in patients with shock of any cause.Reference Sakr, Reinhart and Vincent 22 Another large, multicenter, double-blinded randomized controlled trial showed equivalence between norepinephrine and epinephrine; however, epinephrine was associated with adverse metabolic effects, such as lactic acidosis, tachycardia, and increased insulin requirements.Reference Myburgh, Higgins and Jovanovska 20 Lastly, a recent Cochrane Review on vasopressors for shockReference Havel, Arrich and Losert 14 concluded that there was not sufficient evidence to prove that any of the vasopressors were superior to others; however, dopamine appears to increase the risk for arrhythmias. While the ability to demonstrate mortality benefit for a particular vasopressor in undifferentiated shock is unclear, norepinephrine will have less adverse effects during use as compared to other vasopressors.
Recommendation: In undifferentiated shock, a second vasopressor should be added if a goal MAP>70 mmHg is not being achieved. (Conditional)
Rationale: Observational trends from a large, multicenter, double-blinded randomized controlled trial and a large, multicenter, observational cohort study report the addition of a second vasopressor agent in up to 26%Reference Obritsch, Jung and Fish 18 and 54%Reference Sakr, Reinhart and Vincent 22 of cases in all shock types. If other treatment principles are being addressed and the mean arterial pressure goal is still not being met, then the addition of a second vasopressor may be required. Evidence is not strong enough to recommend a particular agent.
Question 7: How should vasopressors and inotropes be administered to ED patients?
Recommendation: Short term vasopressor infusions (<1–2 hours) or boluses via properly positioned and functioning peripheral intravenous catheters are unlikely to cause local complications. (Conditional)
Recommendation: Vasopressor infusions for prolonged periods (>2–6 hours) should preferentially be administered via central venous catheters. (Conditional)
Rationale: 51 papers were identified in the literature from 1940–2012 describing 200 cases of local complications from vasopressor administration.Reference Russell, Walley and Singer 61 , Reference Bunker and Higgins 111 – Reference Kurland and Malach 162 Vasopressors were administered peripherally in 196 of these casesReference Bunker and Higgins 111 – Reference Tran and Finlayson 113 , Reference Kahn, Kress and Hall 115 – Reference Bergmann 158 , Reference Uricchio, Calenda and Cutts 160 – Reference Kurland and Malach 162 , and centrally in four.Reference Russell, Walley and Singer 61 , Reference Davies, Russell and Thompson 114 , Reference Crawford and Haynes 159
The following observations regarding cases where peripherally administered vasopressors resulted in local complications can be made:
Extravasation with no skin/tissue complications occurred in 66 cases, extravasation with skin/tissue necrosis occurred in 20 cases, and skin/tissue necrosis with no extravasation occurred in 110.
Patients with short infusions of vasopressor medications had few complications. Only 1 case (<1%) of skin necrosis occurred <1 hour after peripheral vasopressor administration; 89.4% of reported complications occurred when vasopressor was administered peripherally for >6 hours. Recognizing the limitations of relying on case series and case reports, this data indicates that complications from peripheral administration of vasopressors tend to occur with prolonged infusions.
Complications occurred more commonly when administered in distal sites. 86% of cases with complications when peripheral intravenous site was located in distal extremities (hand, wrist, forearm, saphenous vein, foot). Only 14% of complications occurred when vasopressor was administered in proximal sites (antecubital fossa, external jugular vein, thigh).
There were four cases reported of local complications from vasopressor administration via central lines.Reference Russell, Walley and Singer 61 , Reference Davies, Russell and Thompson 114 , Reference Crawford and Haynes 159 Extravasation with no skin necrosis occurred in two cases, and skin necrosis with no extravasation occurred in two cases. Site of administration was internal jugular in one case and femoral in another (other articles did not report site). The duration of vasopressor infusion was between 6–120 hours. Recognizing the limitations of relying on case series and case reports, this data indicates that there is a relatively low rate of local complications from central administration of vasopressors.
Recommendation: Inotropes can be given via peripheral catheter (short term) or central venous catheters (prolonged period) with a similarly low incidence of local complications. (Conditional)
Rationale: This recommendation is based on three papers,Reference Stier, Bogner and Webster 116 , Reference Reed, Newman and Applefeld 120 , Reference Zucker, Eisinger and Floch 140 all of which were case series or case reports. Five reports of complications local to site administration were described; three from central administrationReference Reed, Newman and Applefeld 120 and two from peripheral administration.Reference Stier, Bogner and Webster 116 , Reference Zucker, Eisinger and Floch 140 These complications included four cases of skin/tissue necrosis and one of extravasation with no tissue damage, and occurred with administration lasting between 16–864 hours. Given the low incidence of reported cases of local complications from either central or peripheral administration, it is reasonable to conclude that inotrope administration either peripherally or centrally is safe.
Recommendation: The administration of vasopressors via intra-osseous lines is safe in adults. (Conditional)
Rationale: While there are papers evaluating speed and general safety of intra-osseous catheters for vascular access, no reports were found specifically describing safety or local complications from intra-osseous infusion of vasopressors in adults. There are three case reports of local complications in children (compartment syndrome, tissue necrosis, osteomyelitis.Reference Moen and Sarwark 163 – Reference Stoll, Golej and Burda 165 However, these occurred in very young children (6 years, 7 month, 3 months) receiving large resuscitative doses of vasopressors, and are not clearly relevant to adults. Given that the intra-osseous compartment is considered a non-compressible central vein, it seems reasonable to conclude that intra-osseous administration of vasopressors may yield a low incidence of complications from the vasopressor itself.
Conclusions
Vasopressor and inotrope use in the Emergency Department for patients in shock is necessary and commonly prescribed. Definitive treatments must not be delayed nor forgotten if using vasopressors or inotropes. These evidence based guidelines are intended to help guide the Emergency Medicine practitioner to the most effective and safest utilization of these medications. A user-friendly algorithm poster for implementation in Canadian EDs is attached (see powerpoint poster slide Appendix 1). Hopefully, ongoing research trials will provide future high level evidence with which to edit and advance these guidelines.
Competing interests: No authors have submitted a financial nor conflict of interest relating to the submission of this manuscript
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/cem.2014.77










