Introduction
Established strategies for cartilage and bone repair, such as autologous chondrocyte transplantation (ACT) (Ref. Reference Brittberg1) and bone grafting (Ref. Reference Heslop, Zeiss and Nisbet2), have reached broad clinical application and yield satisfactory results due to continuous improvement. These therapies, however, require the excision of healthy tissue from a nonlesioned site, necessarily incorporating the disadvantages of additional medical procedures, donor site morbidity and further rehabilitative burden on the patient (Ref. Reference Marlovits3). Repair strategies that are based on autologous bone-marrow-derived stromal cells (BMSC) do not circumvent these problems, but harvesting bone marrow from the iliac crest is generally judged as less invasive (Ref. Reference Nejadnik4). The discovery that multipotent stromal cells can be isolated from lipoaspirates (Ref. Reference Zuk5) and that the number of adherent cells in an equal volume of adipose tissue exceeds the content of bone marrow aspirate by about 300-fold (Refs Reference Estes6, Reference Oedayrajsingh-Varma7, Reference Pittenger8) challenged the assumption that bone marrow would be the most appropriate source for cell-based therapies of skeletal injuries and diseases.
In order to verify whether adipose-derived stromal cells (ASC) represent an easily accessible cell type that may substitute for BMSC completely in cell-based approaches for osteochondral regeneration, they were characterised in terms of in vitro performance (Refs Reference Gronthos9, Reference Zuk10), in vivo localisation (Refs Reference Shi and Gronthos11, Reference Zannettino12) and their ability to differentiate into various mesenchymal cell types (Refs Reference Hwang13, Reference Lin14, Reference Rousseau15, Reference Zhang16). This review summarises current knowledge of ASC and BMSC plasticity and in vivo function, describing similarities and differences between both cell types that have been determined upon expansion. Furthermore, an overview is provided on osteoarticular regenerative approaches that have thus far been conducted using ASC. In summary, data on ASC-based osteoarticular repair strategies indicate that ASC do not possess intrinsic osteochondral potential, such as BMSC, but require reprogramming for in vivo development towards the osteochondral lineage. These observations stress the concept of equivalent mesenchymal progenitors in bone marrow and adipose tissue (Ref. Reference Pittenger8). In view of a long list of successful experimental intervention studies in distinct models, trophic functions of ASC may be more relevant than stem cell potential in mediating osteoarticular repair.
Stemness of BMSC and ASC
Criteria for stem cell definition
Thus far absent from the literature is a comprehensive, general convention that defines intrinsic properties for stem cells of any given tissue (Ref. Reference Rao17). From a functional point of view, a well-accepted interpretation would be that a single stem cell possesses the capacity to build up a physiological, multicellular tissue that is capable of autonomous regeneration in vivo. Specific cellular functions such as asymmetric cell division, prolonged self-renewal and differentiation capacities are needed to fulfil this requirement. Most importantly, in vitro detection of these properties in a particular cell type alone, however, does not necessarily prove stemness. It is self-explanatory that a stem cell only deserves this designation if the observed fundamental capacities represent intrinsic features of the native cell in vivo, rather than being achieved by artificial treatments or molecular reprogramming. These stringent criteria for stem cell definition (Ref. Reference Bianco18) are met by haematopoietic stem cells (HSC), which reconstitute bone marrow when clonally derived HSC are transplanted into lethally irradiated mice (Ref. Reference Becker, Mc and Till19). In the context of osteoarticular repair, BMSC are so far the only entity representing skeletal stem cells, according to this stringent definition. Sacchetti et al. established that clonal BMSC populations are self-renewing and can form an ectopic bone organ after subcutaneous transplantation into immunocompromised mice (Ref. Reference Sacchetti20). This result was further refined by Chan et al. by demonstrating the formation of multicellular bone tissue at other ectopic sites and by unravelling routes of differentiation from a discrete progenitor subpopulation to cell types that either contribute to bone, cartilage or haematopoiesis-supportive stroma within the new bone organ (Ref. Reference Chan21). Besides BMSC and HSC, a plethora of other cell types is commonly designated as stem cells, although evidence for clonal in vivo organ formation without pre-induction is missing. This circumstance also holds true for ASC that, nevertheless, are often referred to as adipose-derived mesenchymal stem cells, despite the fact that the capacity of a single ASC to build up a functional mesenchymal tissue has not been shown to date. Thus, the idea of universal mesenchymal stem cells in a perivascular niche (Ref. Reference Crisan22) with an intrinsic capacity to build up and maintain multiple mesenchymal tissues (Ref. Reference Pittenger8) by a common mesengenic in vivo process is still an unconfirmed hypothesis. Conclusively, BMSC are the only perivascular cells with proven skeletal stem cell characteristics.
Lack of evidence for stem cell characteristics of ASC
Current data do not exclude that ASC may possess stem cell characteristics, according to the stringent criteria outlined above, and results are encouraging that future experiments may support adipose tissue-specific stem cell properties. In vitro characterisation of isolated ASC demonstrated extensive proliferative potential (Ref. Reference Zuk5). The application of standard in vitro differentiation protocols to ASC reflected possession of osteogenic, adipogenic and chondrogenic differentiation capacity (Ref. Reference Zuk5), although the physiological relevance of these assays has been questioned (Ref. Reference Bianco18). Notably, clonal analyses revealed that >2% of cells within expanded ASC cultures exhibit tri-lineage potential in vitro, indicating that typical isolation protocols lead only to a small fraction of ASC with in vitro multilineage potential after artificial treatment (Ref. Reference Zuk10).
Like the most mesenchymal cells (Ref. Reference Alt23), ASC express a cell surface marker profile that is comparable to BMSC (Refs Reference Gronthos9, Reference Mitchell24), fulfilling all requirements that have been suggested as the minimal criteria for defining multipotent mesenchymal stromal cells (Ref. Reference Dominici25). Although most mesenchymal cells also meet these standards (Refs Reference Alt23, Reference Hematti26), a functional equivalence of ASC and BMSC was construed from this classification and subsequent studies focussed on the question of whether ASC exhibit analogous cartilage and bone regeneration capacities. For this purpose, ASC were directly tested for their application to osteochondral in vivo repair approaches; however, the question whether ASC are skeletal stem cells functionally equivalent to BMSC attracted little interest.
From a retrospective point of view, it seems inconsistent that ASC were first assessed for their cartilage and bone regeneration potential before their capacity to build up and maintain a physiological adipose tissue environment was investigated. Although in vitro engineered adipose tissue would have a promising potential for surgical soft tissue reconstruction (Ref. Reference Gomillion and Burg27), strategies using ASC for that purpose are instead clearly outnumbered by approaches that use (pre-)adipocytes (Refs Reference Cho28, Reference Halbleib29, Reference Hemmrich30, Reference Patel31) or even BMSC (Refs Reference Hong32, Reference Mauney, Volloch and Kaplan33, Reference Neubauer34). Beside studies that address in vitro engineering of adipose tissue from ASC (Ref. Reference Vermette35), ASC were seeded in fibrin (Ref. Reference Mizuno36), alginate (Ref. Reference Jing37) or collagen scaffolds (Ref. Reference Lu38) and subjected to an adipogenic pre-induction protocol prior to subcutaneous implantation. As expected after pre-induction, in vivo adipose tissue formation was reported in these studies. Evidence that transplanted clonal ASC can generate adipose tissue in vivo without such a pre-induction is still missing, but such a demonstration would not only be encouraging for their use in adipose tissue engineering but would also further clarify if ASC may indeed represent tissue-specific stem cells distinct from BMSC.
In vitro characteristics of expanded ASC and BMSC
Similar morphological features but different growth behaviour
A thorough review of the literature on in vitro performance of culture-expanded ASC and BMSC reveals strong similarities between stromal cells of both sources, factually overweighing the differences. For instance, no morphological differences were reported to date, and the same spindle-shaped phenotype was frequently described (Refs Reference Diekman39, Reference Shafiee40, Reference Zhou41). Upon isolation, adherent human and mouse ASC seem to exhibit a higher proliferation rate (Refs Reference Zhou41, Reference Dmitrieva42, Reference Kern43, Reference Peng44, Reference Schubert45), but equal growth behaviour compared to BMSC has also been reported (Refs Reference Shafiee40, Reference Danisovic46). In an extended comparison of growth kinetics, Dmitrieva et al. included the fact that the amount of colony-forming cells in the adipose-derived stromal vascular fraction (SVF) exceeds that of the bone marrow nucleated fraction (BM-NC) by at least two orders of magnitude (Refs Reference Dmitrieva42, Reference Kern43, Reference Bourin47). This leads to the result that ASC underwent significantly less population doublings up to the first passage, even if, as usual, more primary BM-NC were initially plated. It could therefore be speculated that signs of senescence only occur later in ASC (Ref. Reference Kern43) because BMSC underwent more cell divisions in the same passage number. However, Dmitrieva et al. showed that ASC indeed possess extended proliferative potential, since more population doublings were observed before cells acquired a senescent phenotype (Ref. Reference Dmitrieva42).
Dissimilar expression of cell surface markers CD106, CD146 and CD34
Extensive analyses have been conducted to map differences between cultured ASC and BMSC with regard to surface marker expression, leading to a reliable picture in which markers distinguish expanded stromal cells of both sources. Again, it is striking that, despite the application of large panels of antibodies (Refs Reference De Ugarte48, Reference Niemeyer49, Reference Pachon-Pena50), just a few CD markers are differently expressed between ASC and BMSC. Multiple reports describe higher expression of CD106 in BMSC (Refs Reference Shafiee40, Reference Kern43, Reference Bourin47, Reference De Ugarte48, Reference Pachon-Pena50, Reference Boeuf and Richter51, Reference Noel52, Reference Rider53), whereas there exist some studies in which no difference was detected (Refs Reference Niemeyer49, Reference Ikegame54). Analogous data exist for CD146, a cell surface marker of pericytes (Ref. Reference Shih55) that has been used to enrich for multipotent cells (Refs Reference Ruetze56, Reference Sorrentino57) and to identify the localisation of multipotent stromal cells in various tissues (Refs Reference Zannettino12, Reference Sacchetti20, Reference Crisan22, Reference Covas58). Adherent stromal cells from bone marrow and adipose tissue both contain a CD146-positive population, but this fraction is about twofold larger in early passage BMSC (Refs Reference Dmitrieva42, Reference Pachon-Pena50, Reference Rider53, Reference Brocher59).
By means of a quality control check that is commonly performed at the beginning of studies, ASC were frequently analysed for CD34-negativity, since the absence of this marker is a prerequisite to meet the minimal criteria for multipotent mesenchymal stromal cells (Refs Reference Dominici25, Reference Bourin47). However, several reports that dealt with a comparison of ASC and BMSC described that adherent ASC included a substantial CD34-positive fraction, whereas BMSC that were analysed in parallel were completely CD34-negative (Refs Reference De Ugarte48, Reference Pachon-Pena50, Reference Noel52, Reference Yoshimura60, Reference Maumus61). In these studies, the selection of adherent cells from the adipose tissue-derived SVF at first led to a twofold enrichment of CD34-positive cells (Ref. Reference Maumus61), followed by a gradual decrease in subsequent passages (Ref. Reference Mitchell24). Nevertheless, a considerable number of CD34-positive cells was still detected in passage 4 (Refs Reference De Ugarte48, Reference Noel52), and Yoshimura et al. even described that after 20 weeks of cultivation, almost 20% of the ASC population was still CD34-positive. These inconsistent data on CD34 expression in ASC cultures may simply reflect that, depending on tissue source and isolation protocol, CD34-positive endothelial cells were occasionally included in primary isolates and gradually disappeared with culture time, due to unfavoured growth conditions. It remains to be determined, however, if this hypothesis or the choice of an antibody of the appropriate subclass (Ref. Reference Bourin47) accounts for the conflicting results. In any case, the general absence of CD34 in BMSC cultures represents another noticeable difference compared to ASC preparations.
Compared to the CD markers that were discussed above, considerably less experimental data indicate differential expression of CD10 (Ref. Reference Bourin47), CD133 (Ref. Reference Shafiee40), CD54 (Ref. Reference De Ugarte48), HLA-ABC (Ref. Reference Rider53) and CD49d/f (Refs Reference De Ugarte48, Reference Pachon-Pena50) between BMSC and ASC. In summary, the most substantiated differences regarding cell surface proteins are lower expression levels of CD106 (VCAM-1) and CD146 (MCAM) in ASC versus BMSC, both of which point to a less angiogenic signature of ASC that reoccurs in their gene expression profile as discussed below.
Reduced osteogenic gene expression signature in ASC
In terms of global gene and protein expression profiling, comparisons using cDNA microarrays and two-dimensional electrophoresis revealed high consistencies between multiclonal ASC and BMSC cultures (Refs Reference Noel52, Reference Hennig62, Reference Lee63). Nevertheless, hierarchical clustering of protein and gene expression data allowed for a separation of ASC and BMSC specimen, possibly due to the observation that Wnt-signalling-associated genes are more abundant in BMSC (Ref. Reference Noel52). Increased expression levels of genes that are associated with osteogenesis were detected in BMSC (Refs Reference Hennig62, Reference Al-Nbaheen64), arguing for a higher degree of osteochondral commitment (Ref. Reference Brocher59). A similar finding was made by mRNA representational difference analysis, where higher ITM2A expression in ASC could be attributed to a lower chondrogenic potential (Ref. Reference Boeuf65). An enrichment of genes that are involved in angiogenic signalling pathways was reported for BMSC (Ref. Reference Monaco66) and confirmed by higher expression of angiogenic markers, such as angiopoietin and vascular endothelial growth factor (VEGF) in comparison to ASC (Ref. Reference Brocher59). On the other hand, ASC were shown to exhibit a more adipogenic gene expression pattern (Ref. Reference Al-Nbaheen64), illustrated by higher expression levels of adiponectin and visfatin (Ref. Reference Brocher59). In line with the higher proliferation rate of ASC, genes involved in mitosis and DNA replication are also up-regulated compared to BMSC (Ref. Reference Nakanishi67).
Reduced performance of ASC in osteochondral in vitro differentiation assays
In line with indications of an intrinsic osteogenic potential of BMSC, exposure to common osteogenic differentiation media induced more mineralisation (Refs Reference Shafiee40, Reference Pachon-Pena50, Reference Im, Shin and Lee68, Reference Liu69), higher alkaline phosphatase activity (Refs Reference Shafiee40, Reference Peng44, Reference Im, Shin and Lee68) and stronger gene expression of osteogenic markers, such as runx2, osteocalcin, osterix, alkaline phosphatase and collagen-1 (Refs Reference Shafiee40, Reference Peng44, Reference Noel52), compared to ASC. In turn, and corresponding to their physiological origin, ASC seem to exhibit a higher affinity to adipogenic differentiation, since inclusion of lipid droplets (Refs Reference Peng44, Reference Pachon-Pena50, Reference Rider53) and expression of the adipogenic marker gene peroxisome proliferator-activated receptor (PPARγ) (Refs Reference Peng44, Reference Rider53) were more intense than in BMSC upon induction. However, similar adipogenic in vitro differentiation capacities of adipose and bone marrow-derived cells were also reported (Refs Reference Noel52, Reference Liu69, Reference Huang70), but no study described a higher adipogenic potential for BMSC. In line with better in vitro osteogenesis, BMSC also showed better performance in common chondrogenesis assays. In vitro differentiation of BMSC in 3D-pellet culture under treatment with TGF-β resulted in more intense collagen-II staining (Refs Reference Diekman39, Reference Danisovic46, Reference Hennig62, Reference Im, Shin and Lee68, Reference Liu69, Reference Huang70), proteoglycan deposition (Refs Reference Diekman39, Reference Danisovic46, Reference Rider53, Reference Hennig62, Reference Im, Shin and Lee68, Reference Huang70) and gene expression of Sox-9 (Ref. Reference Rider53), compared to ASC. Interestingly, the inferior chondrogenic capacity of ASC can be augmented to BMSC levels when BMP-6 is added to the differentiation medium (Refs Reference Hennig62, Reference Hildner71), an observation with obvious relevance for future in vivo applications of ASC.
Comparison of trophic activity
One main path to tissue reconstruction by cell-based therapeutic strategies involves stem cell activity to establish and build new tissue by proliferating and differentiating cells, which are progeny of the implanted cells. A second way to regeneration is the stimulation of endogenous healing capacity by trophic activity of implanted cells, which attract host progenitor cells and organise repair by local and invading cells. Implanted cells may even disappear after this task has been successfully fulfilled. In this second scenario, even transient stem cell activity or differentiation capacity within target tissues may be dispensable as long as trophic activity is high.
Similar to the established trophic role of BMSC (Refs Reference Choi72, Reference Polacek73), cultured ASC were shown to secrete a wide range of proteins (Ref. Reference Skalnikova74) into conditioned media that predominantly exert anti-apoptotic (Refs Reference Saito75, Reference Wei76, Reference Sadat77), immunomodulatory (Refs Reference Park78, Reference Peng79, Reference Puissant80) and angiogenic (Refs Reference Sadat77, Reference Holnthoner81, Reference Rehman82) effects on co-cultured cell types. Extracellular matrix components and secreted enzymes comprised the largest fraction of the secretome, according to mass spectrometry (Refs Reference Chiellini83, Reference Lee84, Reference Zvonic85), but these molecules are unlikely to be heavily involved in the observed paracrine signalling. These effects are instead mediated by secreted cytokines that typically appear in nano- or picomolar concentrations. Corresponding ELISA and multiplex approaches primarily identified VEGF (Refs Reference Lee86, Reference Blaber87, Reference Cai88), hepatocyte growth factor (HGF) (Refs Reference Rehman82, Reference Kilroy89) and insulin-like growth factor-1 (IGF-1) (Refs Reference Sadat77, Reference Wang90) as factors that are responsible for the described intercellular communication. According to this repertoire, both ASC and BMSC can be expected to display trophic functions (Refs Reference Skalnikova74, Reference Caplan and Dennis91), delineating their potential to stimulate bone and cartilage regeneration solely by trophic mechanisms.
In vivo comparison of ASC and BMSC
The extent of the described in vitro differences between ASC and BMSC gives the impression that cells of both sources may fundamentally differ from each other. This point of view must be carefully considered, since in vitro variances may stem from dissimilar donor tissue processing, cell isolation protocols, cell yield and culture methods. In the context of osteochondral regeneration, the proof of in vivo exchangeability of ASC and BMSC is far more important, and aspects of in vivo stem cell activity like trophic activity should be considered, as long as precise healing mechanisms are unclear for the diverse application settings.
Untreated ASC do not form ectopic bone
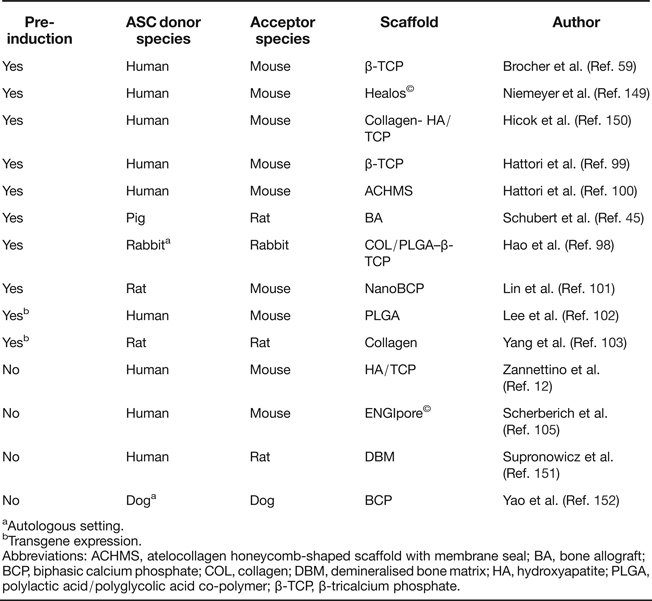
Ectopic bone formation is a standard activity of human BMSC on calcium phosphate ceramics such as β-tricalcium phosphate (β-TCP) and hydroxyapatite (HA)/TCP in immunodeficient mice (Refs Reference Sacchetti20, Reference Janicki92, Reference Kasten93, Reference Janicki94, Reference Kasten95, Reference Krebsbach96, Reference Mankani97) with no osteogenic pre-induction protocols required, in line with skeletal stem cell activity of BMSC. Ectopic transplantation of ASC reliably led to de novo generation of bone when cells were subjected to osteogenic pre-induction before implantation (Refs Reference Schubert45, Reference Hao98, Reference Hattori99, Reference Hattori100, Reference Lin101). Overexpression of BMP-2/RUNX2 (Ref. Reference Lee102) or BMP-7 (Ref. Reference Yang103) in ASC allowed the omission of the pre-differentiation step.
Whether ASC possess the same intrinsic ability to form ectopic bone without any of these pre-treatments in standard assays, and to what extent they build up new bone themselves, largely remained elusive until the issue was recently addressed by Brocher et al. In this first standardised comparison of BMSC and ASC on multiple donors, ASC generated no ectopic bone on osteoconductive scaffolds, while samples from all BMSC donors formed ossicles under identical conditions (Ref. Reference Brocher59). The additional value of this ASC study was the reliable identification of donor as well as host cells within the ectopic setting via a recently established in situ hybridisation technique, specifically marking highly repetitive DNA sequences of human as well as mouse origin (Ref. Reference Steck104). This demonstrated that ectopic bone was formed by ASC when they were subjected to chondrogenic pre-differentiation before transplantation. Although, in noninduced samples, ASC survived at the ectopic site for more than 8 weeks and they did not form bone, as seen in previous studies (Refs Reference Schubert45, Reference Hattori99, Reference Hattori100, Reference Lee102, Reference Scherberich105) (Table 1). Only Zannettino et al. have provided convincing data of ectopic bone formation after ASC implantation on HA/TCP in NOD/SCID mice, when CD146-pre-sorted cells were transplanted; however, the origin of bone from donor or host remained unclear (Ref. Reference Zannettino12).
Table 1. Summary of ectopic bone formation studies with ASC

aAutologous setting.
bTransgene expression.
Abbreviations: ACHMS, atelocollagen honeycomb-shaped scaffold with membrane seal; BA, bone allograft; BCP, biphasic calcium phosphate; COL, collagen; DBM, demineralised bone matrix; HA, hydroxyapatite; PLGA, polylactic acid/polyglycolic acid co-polymer; β-TCP, β-tricalcium phosphate.
All in all, beyond their reduced performance in osteochondral in vitro differentiation assays, ASC showed no intrinsic osteochondral in vivo differentiation potential and, thus, seem to possess no skeletal stem cell properties as seen with BMSC, providing a strong argument for fundamental functional differences regarding their use for in vivo osteochondral repair. Since nonclonal cells are widely used for tissue regeneration, the benefit of enhanced availability of ASC, therefore, appears currently to be balanced by an enhanced need for inductive conditions via timely and intensive in vitro culture efforts, if their physical contribution to the new skeletal tissue is desired.
ASC and BMSC require pre-differentiation for ectopic cartilage formation
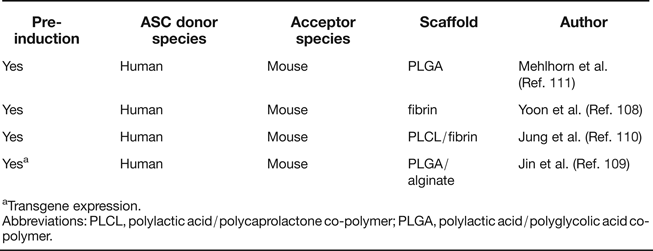
The most convincing demonstration of spontaneous chondrogenic in vivo potential of ASC and BMSC derives from observations of ectopic cartilage deposits in assays, in which articular chondrocytes form cartilaginous nodules at ectopic sites in the absence of chondrogenic inducers. Importantly, such activity has so far neither been demonstrated in human BMSC nor ASC, and an apparent necessary aspect of common strategies for successful ectopic cartilage formation includes chondrogenic pre-differentiation. Several studies have been performed in which human ASC were either used without scaffolds (Refs Reference Hennig62, Reference Dickhut106) or seeded on hydrogels (Refs Reference Dickhut106, Reference Jin107, Reference Yoon108) or glycolic acid/lactic acid copolymer (PLGA) (Refs Reference Jin109, Reference Jung110, Reference Mehlhorn111). In addition, different strategies were used for chondrogenic pre-differentiation of ASC (Table 2). All included TGF-β treatment either during 3D pellet culture (Refs Reference Hennig62, Reference Jin107, Reference Yoon108), in vitro cultivation of ASC in the scaffold (Refs Reference Jung110, Reference Mehlhorn111) or by adenoviral TGF-β overexpression (Ref. Reference Jin109) before subcutaneous implantation into immunocompromised mice. Ectopic cartilage composed of implanted cells was observed in all cases, but chondrocyte hypertrophy and matrix calcification were unwanted side effects reminiscent of growth plate chondrocytes (Refs Reference Hennig62, Reference Pelttari112). In conclusion, analogous studies without chondrogenic (pre-) induction have remained unsuccessful, with neither BMSC nor ASC displaying intrinsic chondrogenic potential nor trophic activity leading to generation of ectopic cartilage of donor or host origin, compared to chondroprogenitors from cartilage which do display such activity (Ref. Reference Dowthwaite113).
Table 2. Summary of ectopic cartilage formation studies with ASC

aTransgene expression.
Abbreviations: PLCL, polylactic acid/polycaprolactone co-polymer; PLGA, polylactic acid/polyglycolic acid co-polymer.
Missing evidence for physical ASC contribution to the repair of damaged cartilage
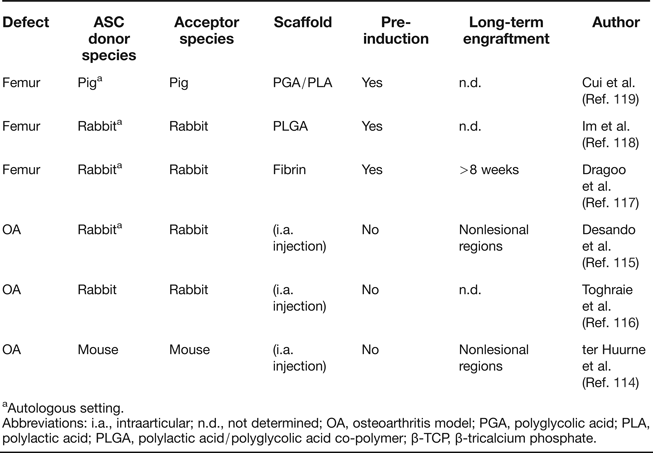
The most direct and least invasive approach to use ASC for the treatment of cartilage defects is by intra-articular injection of cells. Studies that started with an induction of osteoarthritis (OA) by anterior cruciate ligament transection (ACLT) or collagenase treatment, followed by intra-articular injection of autologous ASC, have been conducted in mouse (Ref. Reference ter Huurne114) and rabbit (Refs Reference Desando115, Reference Toghraie116) (Table 3). Different histological evaluations and OA scoring scales were used to measure OA progression, but in all cases, positive effects of ASC compared to the injection of cell-free solvent were reported. Labelled ASC were detectable in the synovial membrane and medial meniscus 20 days after injection (Ref. Reference Desando115) and at the synovial lining and cruciate ligaments up to 5 days after injection (Ref. Reference ter Huurne114). Human ASC injected into unimpaired mouse knee joints showed long-term persistence in joint tissue in 60% of all mice up to 186 days after injection, but a substantial fraction of ASC seemed to have migrated to the bone marrow, adipose tissue and muscle. Thus, while a certain degree of persistence of injected cells can therefore be assumed, evidence for in vivo differentiation of donor ASC or long-term integration into articular cartilage tissue is missing, and contributions by trophic activity cannot be judged.
Table 3. Summary of orthotopic cartilage formation studies with ASC

aAutologous setting.
Abbreviations: i.a., intraarticular; n.d., not determined; OA, osteoarthritis model; PGA, polyglycolic acid; PLA, polylactic acid; PLGA, polylactic acid/polyglycolic acid co-polymer; β-TCP, β-tricalcium phosphate.
Besides artificial OA induction, the capacity of ASC to repair surgical cartilage incisions has been investigated (Table 3). In a scheme similar to ACT, ASC were first harvested, expanded and subjected to chondrogenic pre-induction for 2–3 weeks in vitro. Cells were then loaded on diverse scaffolds and re-implanted into the lesion site. Corresponding studies were conducted in rabbit (Refs Reference Dragoo117, Reference Im, Kim and Lee118) and pig (Ref. Reference Cui119) and differ in pre-induction methods and scaffold composition, but improved healing was consistently reported compared to cell-free matrix baseline conditions. Among these studies, only Dragoo et al. analysed the persistence of donor cells, making use of ASC expressing a LacZ reporter gene (Ref. Reference Dragoo117). All explants showed ASC remaining 8 weeks after transplantation, but their direct contribution to cartilage tissue was not investigated. Notably, approaches for cartilage regeneration without in vitro pre-induction of ASC have so far not been described, although BMSC were successfully used in such a setting (Refs Reference Matsumoto120, Reference Wilke, Nydam and Nixon121, Reference Lim122). Thus, the question of functional equivalence of BMSC and ASC in cartilage repair studies cannot be judged from the current literature, and a direct comparison of both cell sources under identical conditions is highly desired. Importantly, a lack of osteochondral commitment of expanded ASC, although negating their skeletal stem cell activity, may not be a major disadvantage if high trophic activity is paramount for therapeutic action for tissue regeneration and can be achieved with this cell type.
Site-dependant bone repair capacity of ASC
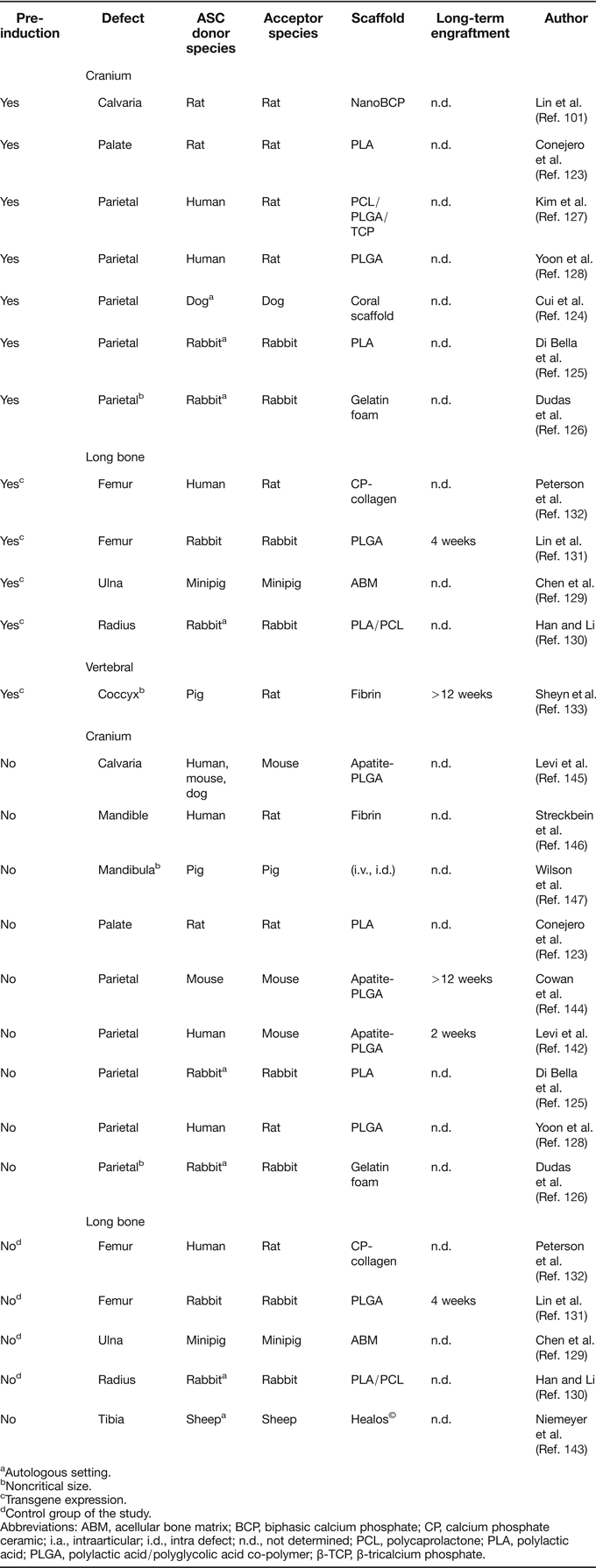
The majority of ASC-based tissue engineering approaches are directed at orthotopic in vivo formation of bone (Table 4). Across all of these studies, a well agreed upon point is that repair of defective bone by ASC can be achieved when transplantation is preceded by extensive pre-differentiation protocols (Refs Reference Lin101, Reference Conejero123, Reference Cui124, Reference Di Bella, Farlie and Penington125, Reference Dudas126, Reference Kim127, Reference Yoon128) or genetic manipulation with genes encoding for bone inducers (Refs Reference Chen129, Reference Han and Li130, Reference Lin131, Reference Peterson132, Reference Sheyn133). Overexpression of BMP-2 represents the most common strategy for the latter technique, using transgenic ASC for local growth factor delivery, rather than expecting spontaneous differentiation into osteoblasts. BMP-2 overexpression in ASC has also been used to substitute strategies that include immobilisation of recombinant BMP-2 protein to scaffolds prior to implantation (Refs Reference de Guzman134, Reference Teixeira and Urist135, Reference Wurzler136). Although these cell-free approaches reproducibly led to good healing efficiency, combinations of ASC and recombinant BMP-2 were also used (Refs Reference Chou137, Reference Keibl138, Reference Mesimaki139, Reference Sandor140, Reference Smith141, Reference Levi142) (not listed in Table 4), but only Levi et al. described that ASC improved defect repair in comparison to the BMP-loaded scaffold alone (Ref. Reference Levi142).
Table 4. Summary of orthotopic bone formation studies with ASC

aAutologous setting.
bNoncritical size.
cTransgene expression.
dControl group of the study.
Abbreviations: ABM, acellular bone matrix; BCP, biphasic calcium phosphate; CP, calcium phosphate ceramic; i.a., intraarticular; i.d., intra defect; n.d., not determined; PCL, polycaprolactone; PLA, polylactic acid; PLGA, polylactic acid/polyglycolic acid co-polymer; β-TCP, β-tricalcium phosphate.
Controversial data exist regarding the performance of expanded but otherwise untreated ASC in the context of long bone repair, although only a limited number of studies is available (Table 4). When nontransgenic or mock-transduced ASC were loaded on carrier matrices and transplanted into long-bone defects, no bone formation was observed (Refs Reference Chen129, Reference Lin131, Reference Peterson132). In a sheep long-bone model, ASC were unable to induce defect bridging, while BMSC facilitated defect regeneration in the same setting (Ref. Reference Niemeyer143). A closer look at the ASC control groups of the above studies further confirms the impression that pre-differentiation or genetic manipulation of ASC is a prerequisite for stimulation of bone formation. This applies even for orthotopic sites in long bones, where the microenvironment is rich in osteoinductive proteins which are released from the defect endings. To our knowledge, the only exception when nontransduced ASC led to substantial bone formation in the context of long-bone repair is a study by Han and Li, in which ASC were used as a control to Runx2-overexpressing cells. Possibly, the surgical connection of the implant to the vascular network was the key to the positive results of this study (Ref. Reference Han and Li130).
Dissimilar to long-bone repair, healing of critical size defects in the cranium appears to be less challenging with untreated ASC, since bone formation without any in vitro pre-differentiation was reported in at least four studies (Refs Reference Cowan144, Reference Levi145, Reference Streckbein146, Reference Wilson147). Furthermore, untreated ASC that were transplanted as controls for newly established repair strategies also generated considerable amounts of bone (Refs Reference Di Bella, Farlie and Penington125, Reference Yoon128, Reference Levi142), although complete absence of defect repair by ASC control groups has also been described (Refs Reference Conejero123, Reference Dudas126). Thus, orthotopic bone formation by uninduced ASC appears to be site-dependent and favoured by characteristics of the cranial microenvironment that are not present in long bones. Origin from the ectodermal germ layer, development via the intramembranous pathway and enhanced blood supply differentiates bone in the cranium from long bones. Thus, it is tempting to speculate that one major advantage in the cranium may be the denser vascular network of skull bones, which is especially interesting in the context that, beyond an absent osteochondral commitment, a lower angiogenic signature was noted for ASC (Refs Reference Dmitrieva42, Reference Brocher59, Reference Monaco66, Reference Kachgal and Putnam148) and orthotopic bone formation by ACS can be triggered by co-transplantation of endothelial cells (Ref. Reference Kim127).
If the trophic activity of ASC is the most crucial for stimulation of bone repair, a lower requirement for attraction and stimulation of endothelial progenitors could explain the better performance of ASC in the cranium. In line with this, a persistence of donor ASC could not be detected for more than 2 to 4 weeks after transplantation, even in settings where complete cranial defect repair was observed (Refs Reference Lin131, Reference Levi142). In sharp contrast, a single study by Cowan et al. reported that transplantation of uninduced ASC led to stable engraftment of the cells in a cranial defect and over 95% of nuclei in the newly formed bone were donor-derived after 12 weeks (Ref. Reference Cowan144). As it is not apparent which specific experimental parameters have enabled this exceptional engraftment, analogous success is waiting for repetition. Overall, particular success of ASC in cranial but not long-bone defects suggests that, in view of their low osteochondral and angiogenic signature, ASC affect bone regeneration most probably via their trophic activity than by in situ differentiation to osteoblasts with long-term persistence. Additional precise studies must unravel the contribution of host and donor cells to tissue repair as well as the influence of scaffolds, pre-cultivation, species and defect site in order to reach consensus on the main mechanisms driving ASC-dependent promotion of osteoarticular repair despite lower osteogenic and angiogenic signatures and an apparent lack of skeletal stem cell properties.
Conclusion
More than 50 in vivo studies have been performed to date in order to verify the potential of ASC to be used for osteoarticular regeneration. In each of the quite heterogeneous experimental setups, specific protocols were established that either enabled chondrogenic or osteogenic differentiation of the cells or that resulted in positive effects on defect healing. Regarding the greater accessibility of ASC compared to BMSC, these data are entirely encouraging for the future use of ACS in skeletal regenerative medicine. However, it is now clear that ASC do not exhibit the same degree of osteoarticular predetermination as BMSC and more manipulation is required to drive ASC into the chondrogenic or osteogenic lineage (Fig. 1). The observations that the spontaneous formation of an ectopic bone organ by BMSC cannot be reproduced with ASC and that orthotopic bone formation is only stimulated at favoured sites confirm this issue and thereby exclude a skeletal stem cell identity for ASC. Altogether, a review of the literature suggests that mainly trophic functions determine the therapeutic outcome after ASC application. Future research is needed on a direct comparison of BMSC and ASC in osteoarticular therapy to decide where and how successful BMSC protocols have to be modified to achieve promising results with ASC.

Figure 1. In vivo cartilage and bone formation potential of ASC: site dependence and requirement for pre-differentiation.
Financial Support
This work was supported by grants from the German Research Foundation, reference numbers RI707/7-1, RI707/8-1 and BO3661/1-1.
Conflicts of Interest
None.