Dietary and endogenous proteins that enter the large intestine of humans or monogastric animal species can be fermented by indigenous bacteria to form metabolites such as NH3, hydrogen sulphide, branched-chain fatty acids, phenols and indols, which may promote intestinal disorders( Reference Blaut and Clavel 1 – Reference Rist, Weiss and Eklund 4 ). For example, protein level and quality in pig diets have been shown to be related to diarrhoea and shedding of enterotoxigenic Escherichia coli after a challenge( Reference Wellock, Fortomaris and Houdijk 5 , Reference Opapeju, Krause and Payne 6 ). A promising approach to reduce the formation of harmful metabolites from protein fermentation in the large intestine is the inclusion of fermentable carbohydrates in the diet( Reference Rist, Weiss and Eklund 4 , Reference De Lange, Pluske and Gong 7 , Reference Heo, Opapeju and Pluske 8 ). This approach can be used to direct bacterial fermentation activity towards utilisation of carbohydrates instead of proteins, yielding lower luminal concentrations of branched-chain fatty acids and NH3 and higher levels of SCFA.
Among SCFA, butyrate is of specific interest, as it serves as a source of energy for colonocytes and plays a crucial role in the epithelial maintenance of gut barrier function( Reference Plöger, Stumpff and Penner 9 ). In addition to non-ionic diffusion, carrier-mediated uptake of butyrate from the large-intestinal lumen can also occur through monocarboxylate transporter 1 (MCT1) expression. Increased formation of butyrate from the fermentation of carbohydrates has been shown to increase the expression of MCT1 in pigs( Reference Metzler-Zebeli, Gänzle and Mosenthin 10 , Reference Haenen, Zhang and Souza da Silva 11 ). MCT1 can also be induced by ketone bodies, pyruvate and lactate in addition to butyrate( Reference Ritzhaupt, Wood and Ellis 12 , Reference Cuff, Lambert and Shirazi-Beechey 13 ). Activation by butyrate probably occurs through a butyrate response element in the promoter region of the MCT1 gene( Reference Borthakur, Saksena and Gill 14 ). In contrast, the expression of the MCT1 gene is down-regulated in colonocytes during transition to malignancy( Reference Lambert, Wood and Ellis 15 ). Moreover, interferon γ (IFN-γ) and TNF-α signalling down-regulates MCT1 expression during inflammation, leading to butyrate deficiency in intestinal epithelial cells( Reference Thibault, de Coppet and Daly 16 ).
Whether metabolites derived from intestinal protein fermentation could influence the expression of MCT1 in pigs is yet unknown. We have previously demonstrated that increased formation of protein-derived metabolites in the large intestine increases the expression of pro- and anti-inflammatory cytokines, tight junction proteins and epithelial response to histamine( Reference Pieper, Kröger and Richter 17 – Reference Richter, Pieper and Zakrzewski 19 ). Of these activities, the pro-inflammatory response may suggest that MCT1 expression is potentially down-regulated by high-protein diets. We hypothesised that such down-regulation of MCT1 expression, if present, could be dependent on the levels of both dietary protein and fermentable carbohydrates, because these nutrients induce microbial metabolites that, in turn, influence cytokine expression. In addition to testing this core in vivo hypothesis, we also used ex vivo (Ussing chamber) and in vitro (Caco-2 cells) approaches to further study the influence of NH3 and TNF-α on the expression patterns of MCT1 and cytokines.
Materials and methods
All procedures involving pig handling and treatments were approved by the State Office of Health and Social Affairs ‘Landesamt für Gesundheit und Soziales Berlin’ (LAGeSo Reg. no. 0389/12).
Animals, diets and sampling
A total of twenty-four piglets (Euroc × Pietrain) with a mean body weight (BW) of 7·4 (se 1·0) kg were weaned at 25 d of age and randomly assigned to one of four dietary treatment groups in a 2 × 2 factorial arrangement, balancing for sex and BW. Diets (Table 1) were formulated to meet or exceed the nutrient requirements of the weaning pig( 20 ). The sources of dietary crude protein (CP) were soyabean meal, fishmeal and potato protein. Fermentable carbohydrates were supplied as sugarbeet pulp (SBP). Water and feed were provided ad libitum. Feed intake and BW of pigs were recorded weekly, and health status was checked daily.
Table 1 Ingredients and chemical composition of the experimental diets

CP, crude protein; SBP, sugarbeet pulp.
* Mineral and vitamin premix (Spezialfutter Neuruppin GmbH) containing per kg DM: 130 g Na (as NaCl); 55 g Mg (as magnesium oxide); 210 mg retinol; 3000 μg cholecalciferol; 8000 mg dl-α-tocopherol; 300 mg menadione; 250 mg thiamin; 250 mg riboflavin; 400 mg pyridoxine; 2000 μg vitamin B12; 2500 nicotinic acid; 100 mg folic acid; 25 000 μg biotin; 1000 mg pantothenate; 80 000 mg choline chloride; 5000 mg Fe (as iron (II) carbonate); 1000 mg Cu (as copper (II) sulphate); 5000 mg Zn (as zinc oxide); 6000 mg Mn (as manganese (II) oxide); 45 mg I (as calcium iodate); 35 mg Se (as sodium selenite).
Piglets were euthanised on experimental day 21 ± 1 by an intracardial injection of 10 mg/kg BW of tetracaine hydrochloride, mebezonium iodide and embutramide (T61®; Intervet) after sedation with 20 mg/kg BW of ketamine hydrochloride (Ursotamin®; Serumwerk Bernburg AG) and 2 mg/kg BW of azaperone (Stresnil®; Janssen-Cilag). Following euthanasia, digesta contents and tissue samples from the colon ascendens were taken and immediately stored at − 80°C until further analysis.
Chemical analyses
Weende proximate nutrients (ash, CP and diethyl ether extract) and starch were determined using standard procedures( Reference Naumann and Bassler 21 ). Total dietary fibre was analysed using a commercial kit (Megazyme K-TDF; Megazyme), as described previously( Reference Pieper, Boudry and Bindelle 22 ). Trace mineral content in feedstuff was determined by atomic absorption spectrometry using an AAS Vario 6 spectrometer (Analytik Jena) after hydrolysis of samples in concentrated HCl.
d- and l-Lactate, NH3, SCFA, biogenic amines, phenols and indols were determined in the digesta samples, as described previously( Reference Pieper, Kröger and Richter 17 , Reference Pieper, Boudry and Bindelle 22 , Reference Pieper, Neumann and Kröger 23 ).
Ussing chamber experiments
Segments of the ascending colon from six piglets (age 54 (se 2) d) fed a standard weaning diet (containing 18 % CP) were collected immediately after euthanasia and settled in a pre-warmed modified Ringer buffer solution, oxygenated with 95 % O2–5 % CO2. The composition of the buffer was 111 mm-NaCl, 5 mm-KCl, 1·5 mm-CaCl2, 1·2 mm-MgCl2, 0·6 mm-NaH2PO4, 2·4 mm-Na2HPO4, 25 mm-NaHCO3, 10 mm-glucose and 2 mm-mannitol, and the pH was adjusted to 7·4. The epithelium was stripped from the serosa and muscular layers, and subsequently mounted on Ussing chambers (exposed area 1·31 cm2). For each pig, six chambers were prepared with two chambers per pig (including control) assigned to each treatment, resulting in a total of six chambers with tissues from three pigs per treatment. The apical and basolateral surfaces of the tissues were rinsed with 15 ml of oxygenated buffer solution and maintained at 37°C in water-jacketed reservoirs. Electrical measurements were obtained using a microcomputer-controlled voltage/current clamp (K. Mussler Scientific Instruments), as described previously( Reference Kröger, Pieper and Schwelberger 18 ). The transepithelial potential difference in response to bipolar 50 μA current pulses generated for 200 ms, and tissue conductance (G t) was calculated according to Ohm's law. After equilibration for approximately 15–30 min, tissues were short-circuited by clamping the voltage at 0 mV. The test substrates Na-butyrate and NH4Cl were added to the mucosal and TNF-α to the serosal side of the tissue to give a final concentration of 20 mmol/l, 20 mmol/l and 50 ng/ml, respectively, with osmolality adjusted to 295 (se 10) mOsmol/l. After 1 h of incubation, the exposed portion of the tissue was harvested and immediately stored in RNAlater RNA Stabilization Reagent (Qiagen GmbH) at − 80°C until use.
Caco-2 cell experiments
To study the influence of different concentrations of Na-butyrate, NH4Cl and TNF-α on the expression of MCT1 and cytokines, in vitro cell-culture experiments using Caco-2 cells were performed. Caco-2 cells were grown routinely in a T-75 plastic flask at 37°C in an atmosphere of 5 % CO2, and maintained in Eagle's minimum essential medium (LGC Standards) supplemented with 20 % fetal bovine serum, 100 units penicillin/ml and 100 μg streptomycin/ml (Biochrom). Confluent Caco-2 cells were subcultured in a ratio of 1:10 using 0·25 %/0·02 % trypsin–EDTA (Biochrom). For the present experiment, cells were seeded on twenty-four-well plates at a density of 105cells/well and allowed to differentiate for 21 d, with fresh medium replacement three times per week. All cells used in the experiments were between passage numbers 19 and 21. Caco-2 cells were treated with buffer of the same composition as that used for electrophysiological measurements indicated above (pH 7·2). Buffer media contained increasing concentrations of Na-butyrate (10, 20 and 50 mmol/l), NH4Cl (5, 10 and 20 mmol/l) or TNF-α (25, 50 and 100 ng/ml). Osmolality was adjusted to 295 (se 10)mOsmol/l. After 1 h of incubation, cells were rinsed with the buffer solution. The buffer was immediately replaced with RNAlater RNA Stabilization Reagent, the cells were scraped from the surface, and the solution was stored at − 80°C for the analyses of MCT1, IL-8 and TNF-α gene expression.
Gene expression analysis
Analysis of gene expression was performed as described previously with slight modifications( Reference Pieper, Kröger and Richter 17 , Reference Martin, Pieper and Schunter 24 ). Briefly, total RNA from colonic tissue and Caco-2 cells was extracted using the NucleoSpin® RNA II Kit (Macherey-Nagel GmbH & Company KG). RNA quality and quantity were determined on an Agilent 2100 Bioanalyzer (Agilent). Subsequently, 100 ng of total RNA were reverse-transcribed into complementary DNA in a final volume of 20 μl using the SuperScript® III Reverse Transcriptase First-Strand complementary DNA Synthesis System (Invitrogen). The complete reaction mix was incubated for 5 min at 25°C, 60 min at 50°C, and 15 min at 70°C in a Sure Cycler 8800 (Agilent Technologies). Primers for MCT1, TNF-α, IFN-γ, IL-8, 60S ribosomal protein L19 (RPL19), β2-microglobulin, succinate dehydrogenase subunit A (SDHA), β-actin and TATA box-binding protein (TBP) were designed based on published sequences. Porcine (RPL19 and SDHA) and human (β-actin and TBP) housekeeping genes were selected for data normalisation. Primer information and annealing temperatures are summarised in Table 2. Quantitative real-time PCR was performed in a total volume of 25 μl, which contained 12·5 μl Brilliant II SYBR Green QPCR Master Mix with Low ROX (Agilent Technologies), 0·5 μl of each primer (10 μm), 10·5 μl of water and 1 μl complementary DNA. The real-time quantitative PCR was performed on a Stratagene MX3000p (Stratagene) with general cycling conditions as follows: one cycle of denaturation at 95°C for 15 min, followed by forty cycles with 30 s denaturation at 95°C, 30 s annealing and 30 s extension at 72°C. Gene expression data were normalised, and times-fold expression was calculated based on mean C t values of the housekeeping genes using real-time PCR efficiency according to the method proposed by Pfaffl( Reference Pfaffl 25 ).
Table 2 List of the primers used in the present study

A T, annealing temperature; F, forward; R, reverse; SDHA, succinate dehydrogenase subunit A; RPL19, 60S ribosomal protein L19; MCT1, monocarboxylate transporter 1; IFN-γ, interferon γ; TBP, TATA box-binding protein.
Statistical analysis
Data from the in vivo study were analysed using general linear model procedures in SPSS (version 21.0; SPSS, Inc.) with SBP and CP and their interaction as sources of variation. Pearson's correlation analysis was performed to determine the correlation between colonic metabolite concentrations and relative gene expression data in pigs. The effects of increasing concentrations of selected metabolites on gene expression in Caco-2 cells were analysed by ANOVA and polynomial (linear, quadratic and cubic) contrasts, corrected for unequal spacing of treatment concentrations. Electrophysiological data from Ussing chambers and gene expression data from the ex vivo and in vitro experiments were analysed by one-way ANOVA followed by Tukey's honestly significant difference test. For non-normally distributed data, the Kruskal–Wallis test was applied to determine group differences, and groups were separated using the Mann–Whitney test. Differences at P< 0·05 were considered significant. Data are presented as means with their standard errors, unless otherwise stated.
Results
During the in vivo study, all pigs remained in good health condition, and no clinical signs of diarrhoea or health impairment were observed. BW gain, feed intake and final BW did not differ significantly between the treatments, although pigs fed SBP diets had numerically lower BW gain and final BW (data not shown).
Metabolite concentrations in the colonic digesta have been described previously( Reference Pieper, Boudry and Bindelle 22 ). Briefly, pigs fed dietary SBP had higher concentrations of lactate and acetate and lower propionate concentration in the colonic digesta (P< 0·05), whereas pigs fed diets high in CP exhibited higher concentrations of butyrate, NH3 and biogenic amines (P< 0·05). Inclusion of SBP in high-CP diets led to lower concentrations of some protein-derived metabolites, such as NH3, cadaverine, p-cresol and indole (P< 0·05), but the values were still higher as with low-CP diets. Other metabolites such as putrescine, histamine, phenol, 4-ethylphenol or 3-methylindol were unaffected. Lactate concentration was slightly lower (P< 0·10) in high-CP diet-fed pigs, whereas inclusion of both CP and SBP led to slightly higher (P< 0·10) levels of SCFA.
The relative gene expression of MCT1, IL-8, TNF-α and IFN-γ in the colon of pigs fed the different diets is given in Table 3. The expression of MCT1 was lower (P< 0·05) in pigs fed high-CP diets, irrespective of SBP. In contrast, the expression of IL-8, TNF-α and IFN-γ was higher (P< 0·05) in pigs fed high-CP diets. The inclusion of dietary SBP had no effect on these gene expression patterns. In addition, no interaction was observed, indicating that dietary CP level was the main factor that influenced the expression of MCT1, IL-8, TNF-α and IFN-γ, irrespective of dietary SBP inclusion.
Table 3 Relative gene expression (fold change) of monocarboxylate transporter 1 (MCT1), IL-8, TNF-α and interferon γ (IFN-γ) in the colon of pigs fed diets high or low in dietary crude protein (CP) with or without the addition of sugarbeet pulp (SBP) (Mean values with their standard errors; n 6)
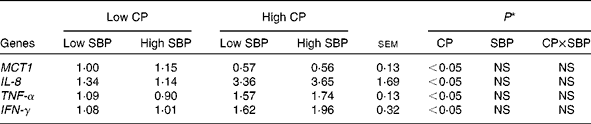
* P values indicate the main effects for CP and SBP, respectively.
Pearson's correlation analysis was performed to determine the correlation between the metabolite concentrations in the colonic digesta and the relative gene expression of MCT1, IL-8, TNF-α and IFN-γ in colonic tissue (Table 4). A positive correlation (P< 0·05) occurred between MCT1 expression and l-lactate concentration, whereas NH3 and putrescine concentrations were negatively correlated with MCT1 expression (P< 0·05). IL-8 gene expression was negatively correlated with l-lactate (P< 0·05) and positively linked with NH3, putrescine, phenol and 4-ethylphenol (P< 0·05). TNF-α expression also negatively correlated with l-lactate and positively correlated with acetate, NH3, putrescine and histamine (P< 0·05). Finally, IFN-γ expression was only positively correlated with histamine and 4-ethylphenol concentrations (P< 0·05).
Table 4 Pearson's correlation of the microbial metabolite concentrations in the colonic digesta with the relative gene expression of monocarboxylate transporter 1 (MCT1), IL-8, TNF-α and interferon γ (IFN-γ) in the colonic tissue of pigs fed diets high or low in dietary crude protein with or without the addition of sugarbeet pulp*
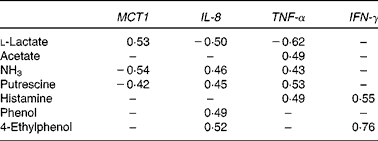
* Only significant correlations (P< 0·05) are indicated.
Colonic tissue from pigs fed commercial standard diets was mounted on Ussing chambers and mucosally exposed to different metabolites. Changes in short-circuit current were found to be higher (P< 0·05) after the mucosal addition of NH4Cl compared with control chambers and chambers treated with Na-butyrate and TNF-α, where practically no biologically relevant change occurred (data not shown). The expression of MCT1, IL-8, TNF-α and IFN-γ was subsequently analysed. MCT1 gene expression was higher (P< 0·05) after Na-butyrate treatment and lower (P< 0·05) after exposure to NH4Cl and TNF-α compared with the untreated controls (Fig. 1(A)). The expression of IL-8 was only increased (P< 0·05) after exposure to TNF-α, whereas all the other treatments had no effect (Fig. 1(B)). The expression of TNF-α was only increased (P< 0·05) after tissue exposure to NH4Cl, whereas Na-butyrate or TNF-α had no effect (Fig. 1(C)). Finally, Na-butyrate decreased (P< 0·05) IFN-γ gene expression, whereas all the other treatments did not (Fig. 1(D)).

Fig. 1 Relative gene expression (fold change) of (A) monocarboxylate transporter 1 (MCT1), (B) IL-8, (C) TNF-α and (D) IFN-γ in colonic tissue after the mucosal addition of Na-butyrate, NH4Cl or TNF-α, respectively. Values are means, with their standard errors represented by vertical bars. a,bMean values with unlike letters were significantly different (P< 0·05).
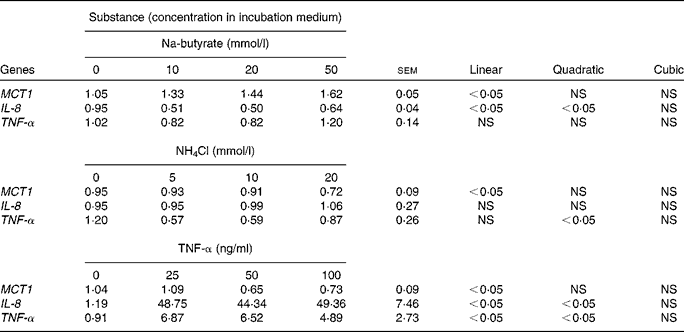
Increasing concentrations of Na-butyrate, NH4Cl and TNF-α were used to study the possible dose-dependent effects in Caco-2 cells (Table 5). The expression of MCT1 was increased with Na-butyrate treatment and decreased with NH4Cl and TNF-α treatments (linear, P< 0·05), respectively. Linear and quadratic effects (P< 0·05) were also observed for IL-8 gene expression after Na-butyrate and TNF-α treatments, respectively. Similarly, TNF-α gene expression showed a quadratic response (P< 0·05) towards increasing concentrations of NH4Cl, and a linear and quadratic response (P< 0·05) after exposure to TNF-α.
Table 5 Relative gene expression (fold change) of monocarboxylate transporter 1 (MCT1), IL-8 and TNF-α in Caco-2 cells after the addition of different concentrations of Na-butyrate, NH4Cl or TNF-α, respectively (Mean values with their standard errors; n 6).
Discussion
Previous studies( Reference Metzler-Zebeli, Gänzle and Mosenthin 10 , Reference Haenen, Zhang and Souza da Silva 11 , Reference Woodward, Regmi and Gänzle 26 ) have shown that diets high in resistant starch or soluble fibres up-regulate MCT1 expression in the large intestine of pigs. However, to date, the influence of dietary protein and protein-derived bacterial metabolites on MCT1 expression is largely unknown. In the present study, high CP dietary level led to the down-regulation of MCT1 expression in the colon of pigs, irrespective of dietary SBP inclusion. The fact that no interaction between CP and SBP was observed could be either due to data variability or the number of animals per treatment group (n 6). In contrast, inclusion of SBP into high-CP diets did not reduce the levels of protein-derived metabolites to similar levels as with low-CP diets( Reference Pieper, Boudry and Bindelle 22 ). Previous findings have also shown that gene expression of cytokines in the colon increased with high-CP diets, irrespective of dietary fermentable carbohydrate inclusion( Reference Pieper, Kröger and Richter 17 ). This is, to a certain extent, in contrast to our initial hypothesis; however, it can be concluded that in the present study, the decreased expression of MCT1 coincided with increased luminal availability of protein fermentation products such as NH3, and the concomitantly increased pro-inflammatory cytokine expression in the colonic mucosa.
The role of MCT1 in the transport of butyrate into colonocytes has been well studied. MCT1 is present on the luminal membrane of the human and pig colon, and is involved in the uptake of butyrate by both proton co-transport and anion-exchange mechanisms, as has been discussed in the literature( Reference Rist, Weiss and Eklund 4 , Reference Ritzhaupt, Ellis and Hosie 27 , Reference Gill and Dudeja 28 ). It has been demonstrated that butyrate uptake is enhanced at acidic pH and inhibited or reduced by structural analogues such as acetate, propionate, l-lactate or pyruvate( Reference Rist, Weiss and Eklund 4 , Reference Ritzhaupt, Ellis and Hosie 27 ). In addition to its role as a colonic fuel, butyrate is involved in the maintenance of colonic homeostasis by regulating the expression of genes linked to cellular processes, including proliferation, differentiation or apoptosis( Reference Daly and Shirazi-Beechey 29 ).
Previous studies on human colonic AA/C1 cells have revealed that butyrate increases MCT1 mRNA and protein expression( Reference Cuff, Lambert and Shirazi-Beechey 13 ). Other studies have shown that MCT1 promoter activity is stimulated by Na-butyrate in a dose-dependent way, and that this is related to the NF-κB signalling pathway( Reference Borthakur, Saksena and Gill 14 ). Coherent with these results, earlier studies have found higher MCT1 expression in pigs fed high levels of fermentable carbohydrates that promoted higher levels of colonic butyrate( Reference Metzler-Zebeli, Gänzle and Mosenthin 10 , Reference Woodward, Regmi and Gänzle 26 ). However, the sole inclusion of SBP to a diet without an increase in colonic butyrate concentration was not able to increase MCT1 expression in the present study. In contrast to previous studies where SBP inclusion stimulated butyrate formation in the colon( Reference Anguita, Gasa and Nofrarias 30 , Reference Molist, Gómez de Segura and Gasa 31 ), such an increase was not observed in the present study where only lactate and acetate concentrations were affected by the inclusion of dietary SBP.
If butyrate specifically promotes MCT1 expression in the colon, the question arises why higher butyrate absorption was not accompanied by an increment in MCT1 expression when induced by a diet high in CP. Although SCFA are mainly derived from saccharolytic fermentation, they can also be derived from amino acids, such as lysine or histidine, through reductive deamination( Reference Smith and Macfarlane 32 ). However, apart from butyrate, our diets high in CP also increased the luminal concentrations of NH3 and biogenic amines. The latter were inversely associated with the expression of MCT1, and positively correlated with pro-inflammatory cytokines. An up-regulation of pro- and anti-inflammatory cytokines in the colon of pigs has also been observed in a previous study when high-protein diets were fed( Reference Pieper, Kröger and Richter 17 ). Interestingly, chronic intestinal inflammation (inflammatory bowel disease) is well known to coincide with reduced MCT1 expression in the intestinal mucosa( Reference Thibault, de Coppet and Daly 16 ). Furthermore, treatment with the pro-inflammatory cytokines TNF-α and IFN-γ in colonic HT29 cells down-regulated MCT1 mRNA and protein expression( Reference Thibault, de Coppet and Daly 16 ). The present results suggest that NH3 and other protein-derived metabolites present in the lumen may entail inflammatory responses in the colonic mucosa, which negatively influence the expression of MCT1. This may provide an interesting link between impaired protective effects of butyrate on the colonic epithelium and pro-inflammatory conditions in the colon of pigs fed high-protein diets. In fact, the higher butyrate concentration in the colonic lumen of piglets fed a high-protein diet might, in part, even be a consequence of decreased butyrate absorption due to decreased MCT1 expression. When assuming that butyrate is accumulated in the lumen because of decreased uptake by colonocytes, it follows that the intracellular concentration of butyrate in colonocytes might have been unchanged or even lower on a high-protein diet despite higher luminal concentrations. Such concept of ‘cellular butyrate starvation’ upon inflammatory down-regulation of MCT1 expression is well established as a mechanism contributing to the progression of inflammatory bowel disease( Reference Thibault, de Coppet and Daly 16 ).
To further assess the individual effects of NH3, Na-butyrate and TNF-α on the expression of MCT1 and cytokines in the target tissue, the pig colonic mucosa was mounted on Ussing chambers. In agreement with previous reports, Na-butyrate resulted in a higher expression of MCT1, whereas TNF-α had an inhibitory effect( Reference Cuff, Lambert and Shirazi-Beechey 13 , Reference Thibault, de Coppet and Daly 16 ). Butyrate has anti-inflammatory properties in human intestinal epithelial cells, which is probably mediated by the inhibition of the NF-κB pathway( Reference Lührs, Gerke and Schauber 33 , Reference Yin, Laevsky and Giardina 34 ). Furthermore, colonic and systemic immunoreactivity was reduced after a long-term feeding experiment with resistant starch in pigs, possibly due to an increased luminal concentration of butyrate( Reference Nofrarías, Martínez-Puig and Pujols 35 ). In contrast, NH3 is a putatively toxic metabolite derived from amino acid fermentation in the large intestine. Some studies have confirmed the potential toxicity on the gut epithelium with adverse effects on both colonocyte metabolism and barrier function( Reference Blaut and Clavel 1 , Reference Windey, De Preter and Verbeke 3 , Reference Richter, Pieper and Zakrzewski 19 ). There is also evidence for NH3 as a precursor of cancer and inflammatory bowel diseases in humans( Reference Hughes, Magge and Bingham 36 – Reference Blachier, Mariotti and Huneau 38 ). NH3 has been shown to interfere with oxidative metabolism in colonocytes( Reference Darcy-Vrillon, Cherbuy and Morel 39 ). Moreover, it has been associated with a mucosal inflammatory response, which, in turn, can also reduce butyrate oxidation( Reference Nancey, Moussata and Graber 40 ). Therefore, a reduced expression of MCT1 under inflammatory conditions may result in reduced uptake and oxidation of butyrate. In support of the in vivo findings, colonic tissue treated with NH4Cl exhibited lower MCT1 and up-regulated TNF-α gene expression. Similarly, colonic tissue had also reduced MCT1 expression after treatment with TNF-α, while IL-8 was markedly increased in parallel. Although no direct effect of TNF-α on MCT1 promoter activity or butyrate uptake has been reported previously in Caco-2 cells( Reference Borthakur, Saksena and Gill 14 ), the present results reinforce the notion that MCT1 is down-regulated by pro-inflammatory cytokines.
Finally, increasing concentrations of the same substrates were used to study dose-dependent effects in Caco-2 cells. The regulation of MCT1 by Na-butyrate and TNF-α is in agreement with the results obtained from the ex vivo experiments and previous studies( Reference Cuff, Lambert and Shirazi-Beechey 13 , Reference Borthakur, Saksena and Gill 14 , Reference Thibault, de Coppet and Daly 16 ). The stimulation of Caco-2 cells with increasing concentrations of Na-butyrate increased MCT1 expression, whereas treatment with NH4Cl and TNF-α had opposite effects, supporting the idea of the regulation of MCT1 gene expression by protein-derived metabolites such as NH3 and pro-inflammatory signalling. However, the underlying mechanisms need further elucidation.
In conclusion, the present study shows that diets high in CP reduce the expression of MCT1 in the colon of pigs, even when higher concentrations of butyrate are present in the gut lumen. This effect seems to be related to an inflammatory response of the colonic mucosa triggered by metabolites derived from the bacterial fermentation of protein. While we provide data that NH3 is one important fermentation product involved in the down-regulation of MCT1 gene expression, the present correlation analyses from the in vivo study suggest that other metabolites (e.g. putrescine) could have also been involved. Further studies are required to investigate the complete portfolio of luminal protein-derived fermentation products that affect MCT1 gene expression and their precise mechanism of action.
Acknowledgements
The authors are grateful to the staff of the Institute of Animal Nutrition at the Freie Universität Berlin for their excellent support during the animal experiments and laboratory analyses.
The present study was financially supported by the German Research Foundation (DFG) through research grant no. SFB852/1. C. B. was financially supported by the National Fund for Scientific Research (FNRS) and Wallonie-Bruxelles International (WBI; Brussels, Belgium).
The authors' contributions are as follows: C. V. T., C. B., J. Z. and R. P. contributed to the conception and design of the research; C. V. T., C. B. and R. P. performed the experiments and analysed the data; C. V. T. and R. P. drafted the manuscript; C. V. T., C. B., F. S., J. R. A., W. V., J. Z. and R. P. edited and revised the manuscript; C. V. T., J. R. A., W. V., J. Z. and R. P. approved the final version of the manuscript.
None of the authors has any financial or personal conflict of interest.











