Bone diseases such as osteoporosis and osteomalacia are a major cause of excess mortality, morbidity and health and social services expenditure in older individuals, because of their association with low trauma fractures and skeletal deformity. The importance of Ca and vitamin D in the maintenance of bone health has stimulated interest in the potential role of other nutritional factors.
A number of reports and documents have shaped and influenced public health policy on nutrition and bone health in the UK. The UK Department of Health Committee on Medical Aspects of Food and Nutrition Policy (COMA) report on dietary reference values (DRV) in 1991 included nutrients relevant to bone health1. The Department of Health Advisory Group on Osteoporosis report in 1994 suggested that DRV should be reviewed, in the light of more recent studies2. This was undertaken in the Department of Health report on nutrition and bone health in 1998, which particularly concentrated on Ca and vitamin D3. Other nutritional factors were addressed briefly, including body weight, protein, vitamins K and C, Mg, P, Na, K, fluorine, alcohol, caffeine and phyto-oestrogens3.
The Food Standards Agency Expert Group on Vitamins and Minerals published a report on safe upper limits of nutrients in 2003, which included a detailed review of nutritional and toxicological data on thirty-four vitamins and minerals4. Safe upper limits were recommended for eight of the nutrients, whilst guidance was provided for twenty-two vitamins and minerals where the evidence was less clear, including those related to bone health. Ca supplementation up to 1500 mg/d was regarded as safe, as was vitamin D up to 25 μg (1000 IU) daily and vitamin A up to 1500 μg retinol equivalents daily4.
The WHO published a report on diet, nutrition and the prevention of chronic diseases in 20035. The osteoporosis section suggested that in countries with a high fracture incidence, low Ca intake ( < 400–500 mg/d) was associated with increased risk in older individuals. It was suggested that an increase in dietary intake of vitamin D and Ca in this group could reduce fracture risk. If sunshine exposure was limited, a vitamin D intake of 5–10 μg (200–400 IU) daily was also recommended. This report also summarised the evidence that other nutritional factors either increased or decreased the risk of fracture5. Although many studies have examined the effect of nutrition on markers of bone health, such as biochemical markers of bone turnover and bone mineral density (BMD), fracture is the most important outcome measure.
Dietary requirements for calcium
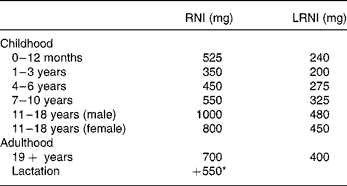
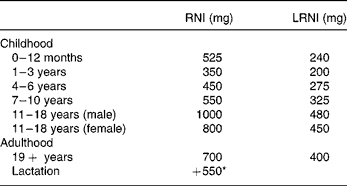
DRV for Ca were set by the Committee on Medical Aspects of Food and Nutrition Policy in 1991, based on factorial calculations which take into account the efficiency of Ca absorption, obligatory losses in the faeces, urine and sweat and the requirements for growth in childhood1. The reference nutrient intake (RNI) of 700 mg/d for adults, which should provide sufficient Ca for 97·5 % of the adult population, is two standard deviations above the estimated average requirement of 550 mg/d. The lower RNI is 400 mg/d, below which dietary Ca is likely to be inadequate, is two standard deviations lower than the estimated average requirement. No other measure of bone health was considered in 1991, but the subsequent report in 1998 examined the effects of high Ca intakes on BMD and fracture incidence in longitudinal and intervention studies3. This report endorsed the earlier DRV (Table 1), but it was felt that the additional 550 mg/d in lactation was probably unnecessary.
Dietary calcium intake in the United Kingdom
The major source of Ca is dairy produce, but other Ca-rich foods include flour, cereals, fish such as sardines and vegetables such as spinach and broccoli. The National Diet and Nutrition Survey showed that 79 % of boys and girls aged 11–14 years have a dietary Ca intake lower than the RNI, with 12 % of boys and 24 % of girls in this age group having an intake below the lower RNIReference Gregory, Lowe, Bates, Prentice, Jackson, Smithers, Wenlock and Farron6. In contrast, only 2 % of men and 5 % of women aged 19–64 years have a dietary intake below the lower RNIReference Henderson, Irving, Gregory, Bates, Prentice, Perks, Swan and Farron7. In older women, the proportion with a Ca intake below the lower RNI increases from 8 % between the ages of 65 and 74 years to 15 % at 85 years and older, whereas the corresponding figures for men are 4 % and 2 % respectivelyReference Finch, Doyle, Lowe, Bates, Prentice, Smithers and Clarke8.
Calcium supplementation
In view of the potentially inadequate dietary Ca intake in children and older individuals, it is important to review the effects of supplementation on bone health. A recently published meta-analysis of fifteen randomised controlled trials involving 2859 children examined the effects of the addition of 300–1200 mg elemental Ca in supplementation or dairy produceReference Winzenberg, Shaw, Fryer and Jones9. The duration of Ca supplementation ranged from 8 months to 4 years and post-supplementation follow up from 8 months to 8 years. This meta-analysis concluded that there was no significant effect of Ca supplementation on femoral neck or lumbar spine BMD. There was a small effect on total body bone mineral content and upper-limb BMD, but it is felt that this was unlikely to influence the risk of subsequent fractures. There is some debate about the appropriateness of using BMD as a single marker of bone health in children and adolescents, where considerations about skeletal mass and size are also requiredReference Prentice, Schoenmakers, Laskey, de Bono, Ginty and Goldberg10.
A meta-analysis of Ca supplementation examined fifteen randomised controlled trials in 1806 postmenopausal women with BMD or fractures as outcome measuresReference Shea, Wells and Cranney11. Ca decreased bone loss by 1·66 (95 % CI 0·92, 2·39) % at the spine, 1·64 (95 % CI 0·70, 2·57) % at the hip and 2·05 (95 % CI 0·24, 3·86) % for total body BMD. Despite this beneficial effect on bone loss, there was no reduction in the risk of vertebral fractures (relative risk 0·77 (95 % CI 0·54, 1·09)) or non-vertebral fractures (relative risk 0·86 (95 % CI 0·43, 1·72)).
The Women's Health Initiative Study of Ca and vitamin D supplementation was performed in 36 282 post-menopausal women aged 50–79 yearsReference Jackson, LaCroix and Gass12. These were randomised to receive Ca (1000 mg) and vitamin D (10 μg; 400 IU) or placebo daily. Although Ca and vitamin D supplementation resulted in a small beneficial effect on bone loss, there was no overall reduction in fractures, clinical vertebral fractures or hip fractures. Post hoc analysis suggested that Ca and vitamin D supplementation decreased the risk of fractures in older individuals and in those who remained compliant with treatment. Nevertheless, any potential benefits were offset by an increased risk of renal stones (hazard ratio 1·17 (95 % CI 1·02, 1·34)). Other studies show that although Ca supplementation increases the risk of renal stones, a high dietary intake of Ca is associated with a lower risk of renal stonesReference Curhan, Willett, Speizer, Spiegelman and Stampfer13.
The results of these studies suggest that the DRV for Ca in the UK are appropriate and do not support the routine use of Ca supplementation in the general population. Nevertheless, a significant proportion of younger adolescents and older individuals have a dietary Ca intake lower than the DRV and may benefit from an increased Ca intake.
Dietary requirements for vitamin D
The major source of vitamin D is cutaneous production, following exposure to UV radiation. The diet generally provides smaller amounts of vitamin D, but this source is essential when cutaneous production is limited, because of lack of exposure to sunlight. The major dietary sources of vitamin D are fortified margarine and other fat spreads, cereals, oily fish, meat eggs and dairy produce. The most important consequence of vitamin D deficiency is the development of osteomalacia, which is characterised by an impairment of bone mineralisation and accumulation of osteoid. The DRV for vitamin D set in 1991 were based on the dietary amount required to ensure that the serum 25-hydroxyvitamin D (25OHD) in winter was above 20 nmol/l, as vitamin D-deficiency osteomalacia generally only occurs in individuals with lower circulating concentrations1. When the DRV for vitamin D were reviewed by the Department of Health Nutrition and Bone Health report in 1998, no changes to the RNI were made3. No RNI was set for children above the age of 3 years or adults below the age of 65 years, unless they were considered at risk of vitamin D deficiency (because of strict dress code, being house-bound or having increased skin pigmentation) when an RNI of 10 μg (400 IU) daily was set. It is important to consider if this RNI is adequate in individuals with no cutaneous production of vitamin D, such as completely veiled women or submariners. In the latter group, vitamin D intakes of 15 μg (600 IU) daily are required to maintain serum 25OHD at its previous levelReference Holick14. As the mean intake of vitamin D from food sources in adults in the UK ranges 2·0–4·0 μg (80–160 IU) dailyReference Henderson, Irving, Gregory, Bates, Prentice, Perks, Swan and Farron7, Reference Finch, Doyle, Lowe, Bates, Prentice, Smithers and Clarke8, most individuals at risk of developing vitamin D deficiency will require supplementation. This should probably be administered as vitamin D3 (cholecalciferol) rather than vitamin D2 (ergocalciferol), as there is evidence suggesting that the metabolism of the latter may be impairedReference Armas, Hollis and Heaney15. In a study of twenty normal healthy men, a single oral dose of 1·25 mg (50 000 IU) of either vitamin D2 or vitamin D3 was administered, following which the change in serum 25OHD was monitored over a 28 d period. Although there were similar increases in serum 25OHD in the first 3 d after the administration of vitamin D2 and vitamin D3, at the end of the study the serum 25OHD was 50 nmol/l above baseline after vitamin D3 administration, but was 5 nmol/l lower than the initial value in the vitamin D2-treated groupReference Armas, Hollis and Heaney15.
Vitamin D deficiency in the United Kingdom
The prevalence of vitamin D deficiency in older individuals in the UK was examined in the National Diet and Nutrition SurveyReference Finch, Doyle, Lowe, Bates, Prentice, Smithers and Clarke8. Using a threshold value of serum 25OHD of < 25 nmol/l, the prevalence of vitamin D deficiency increased from 5–6 % between the ages of 65 and 74 years to 13–25 % above the age of 85 years. The prevalence of vitamin D deficiency in care-home residents was higher than 36 %. Similar findings were reported in the recent Health Survey for EnglandReference Hirani and Primatesta16.
Although classically vitamin D deficiency has been associated with osteomalacia, there has been growing interest in the role of vitamin D insufficiency and secondary hyperparathyroidism in the development of osteoporosis and low trauma fracturesReference Lips17. There is an inverse relationship between serum 25OHD and parathyroid hormone, but no clear evidence of a threshold value of 25OHD above which parathyroid hormone reaches a plateauReference Vieth, Ladak and Walfish18. The relationship between 25OHD and parathyroid hormone may be used to establish a threshold value for vitamin D insufficiency, but there is no clear agreement on the appropriate level to selectReference Dawson-Hughes, Heaney, Holick, Lips, Meunier and Vieth19. This may have been aggravated by the use of different assays for serum 25OHDReference Lips, Chapuy, Dawson-Hughes, Pols and Holick20.
Lips has classified vitamin D insufficiency as mild (serum 25OHD 25–50 nmol/l), moderate (serum 25OHD 12·5–25 nmol/l) and severe (serum 25OHD < 12·5 nmol/l)Reference Lips17. North American experts have suggested that higher levels of 25OHD are essential not only to maintain bone healthReference Dawson-Hughes, Heaney, Holick, Lips, Meunier and Vieth19, but also for mental health, prevention of cancers, cardiovascular health, and for the prevention of skin and autoimmune disordersReference Holick and Jenkins21. Holick & Jenkins suggest that a healthy 25OHD is between 75–150 nmol/l, whilst the minimum for cell health is 75 nmol/l and the minimum for bone health is 50 nmol/lReference Holick and Jenkins21. Relatively few individuals in the UK achieve these levels of 25OHD in winter and spring, when over 80 % of 45-year-old men and women have a serum concentration < 75 nmol/lReference Hypponen and Power22.
Heaney et al. examined the effects of different doses of vitamin D supplementation in sixty-seven men living in Omaha (NE, USA)Reference Heaney, Davies, Chen, Holick and Barger-Lux23. These men were treated with oral vitamin D3 at 0, 25, 125 or 250 μg daily for 20 weeks during the winter. Serum 25OHD measurements were performed regularly during this time. The maximum serum 25OHD achieved with 25 μg daily was approximately 75 nmol/l, whilst the values for 125 μg daily were 140 nmol/l and that for 250 μg was 200 nmol/l. A recent paper reviewed the optimal serum 25OHD concentration suggested by experts and the doses of oral vitamin D3 required to achieve these levelsReference Dawson-Hughes, Heaney, Holick, Lips, Meunier and Vieth19. Heaney et al. suggested that a 25OHD of 80 nmol/l was appropriate, which required an oral vitamin D intake of 40 μg (1600 IU) dailyReference Dawson-Hughes, Heaney, Holick, Lips, Meunier and Vieth19. Although this is higher than the daily upper intake of 25 μg (1000 IU) vitamin D recommended by the Food Standards Agency Expert Group on Vitamins and Minerals4, a recent review suggests that intakes as high as 250 μg (10 000 IU) daily are safeReference Hathcock, Shao, Vieth and Heaney24, at least in the short term.
Vitamin D supplementation
A meta-analysis of vitamin D supplementation published in 2005 indicated that doses of 17·5·20 μg (700–800 IU) daily decreased the risk of hip and other non-vertebral fractures, whereas lower doses (10 μg; 400 IU daily) were ineffectiveReference Bischoff-Ferrari, Willett, Wong, Giovannucci, Dietrich and Dawson-Hughes25. A number of recent studies cast doubt on the role of vitamin D supplementation, with or without Ca, in the prevention of fractures in older individualsReference Jackson, LaCroix and Gass12, Reference Smith, Anderson, Raphael, Crozier and Cooper26–Reference Lyons, Johansen, Brophy, Newcombe, Phillips, Lervy, Evans, Wareham and Stone30. A recent Cochrane reviewReference Avenell, Gillespie, Gillespie and O'Connell31, which includes some of these recent studiesReference Smith, Anderson, Raphael, Crozier and Cooper26–Reference Porthouse, Cockayne and King28, suggests that vitamin D used alone has no significant effect on hip fracture (seven trials; 18 668 participants; relative risk 1·17 (95 % CI 0·98, 1·41)). In contrast, combined supplementation with vitamin D and Ca marginally reduced the risk of hip fractures (seven trials; 10 376 participants; relative risk 0·81 (95 % CI 0·68, 0·96)), but the effect appeared to be restricted to those living in institutional careReference Avenell, Gillespie, Gillespie and O'Connell31. Another recent meta-analysis also shows a similar reduction in hip fractures with Ca and vitamin D (relative risk 0·82 (95 % CI 0·71, 0·94)), with no beneficial effect of vitamin D alone on fracture incidenceReference Boonen, Lips, Bouillon, Bischoff-Ferrari, Vanderschueren and Haentjens32.
Two recent studies have examined the effects of vitamin D in care-home residents, who are likely to be vitamin D deficientReference Law, Withers, Morris and Anderson29, Reference Lyons, Johansen, Brophy, Newcombe, Phillips, Lervy, Evans, Wareham and Stone30. Law performed a cluster randomised trial of oral vitamin D2 (2·5 mg; 100 000 IU) three-monthly in 3717 care-home residentsReference Law, Withers, Morris and Anderson29. Serum 25OHD in a small sub-set increased from 47 to 82 nmol/l after 1 month and 74 nmol/l after 3 months. Despite this improvement in vitamin D status, there was no reduction in falls (relative risk 1·09 (95 % CI 0·95, 1·25)) or fractures (relative risk 1·48 (95 % CI 0·99, 2·20)). A second randomised controlled trial by Lyons et al. examined the effect of oral vitamin D2 (2·5 mg; 100 000 IU) four-monthly in 3440 care-home residentsReference Lyons, Johansen, Brophy, Newcombe, Phillips, Lervy, Evans, Wareham and Stone30. Baseline serum 25OHD measurements were not performed, but during the study these were 80 and 54 nmol/l respectively in a small sub-set of the intervention and control group. There was no reduction in fracture risk with vitamin D supplementation (hazard ratio 0·95 (95 % CI 0·8, 1·20)).
Although there is controversy about what constitutes adequate vitamin D status, the recent studies of vitamin D supplementation suggest that the conservative thresholds adopted in the UK may be more appropriate than those advocated in North America. Combined Ca and vitamin D supplementation may decrease fracture risk in care-home residents, in whom vitamin D deficiency is common, but is probably ineffective in the prevention of fractures in community-dwelling older individuals. Future studies of vitamin D supplementation should include measurements of vitamin D status in a greater proportion of the participants than in previous trials, to investigate the relationship between putative benefits and the baseline and peak serum 25OHD concentrations achieved with supplementation.
Other nutritional factors
The effect of low BMI was examined in a recent meta-analysis in 14 887 men and 44 757 women from twelve prospective cohort studiesReference De Laet, Kanis and Oden33. This showed little effect of BMI on fracture risk between 25 and 35 kg/m2, but the risk of hip fracture increased dramatically as the BMI fell below 25 kg/m2. The relative risk of hip fracture at a BMI of 20 kg/m2was 1·95 increasing to 4·48 at a BMI of 15 kg/m2.
The effect of alcohol consumption was also examined in 5939 men and 11 032 women from three prospective cohort studiesReference Kanis, Johannson and Johnell34. This suggested that the risk of fractures increased in men drinking ≧3 units alcohol/d. Although the overall risk of osteoporotic fractures in women only increased with an intake of ≧4 units/d, hip fractures increased with only 3 units/d.
There has been growing interest in the role of fruit and vegetables in the maintenance of bone health. Studies show a beneficial effect on bone density and bone turnover markers, but there is no evidence yet of a reduction in fracture riskReference Burns, Ashwell and Berry35. It is also currently unclear if the putative benefits of fruit and vegetable consumption are mediated in changes in acid–base balance, vitamin K or the consumption of antioxidants.
Although our knowledge of the effects of nutrition on bone health is gradually increasing, there are still a large number of unanswered questions. It is therefore important to remember Voltaire, who stated that ‘only charlatans are certain… doubt is not a very agreeable state, but certainty is a ridiculous one.’
Acknowledgements
The present paper is based on the opening presentation at the Food Standards Agency Workshop on Nutrition and Bone Health, November 2006. The author is grateful for the helpful comments of participants attending the workshop. The views expressed are those of the author and do not necessarily reflect those of the Food Standards Agency or the workshop participants.