Although breast milk is a valuable food source with confirmed merits, it may not be appropriate in all circumstances(1–Reference Coveney7). Chemicals, particularly ethanol, imbibed in accordance with maternal traditions have drawn attention because they can be passed to nursing infants through breast milk(Reference Argote-Espinosa, Flores-Huerta, Hernandez-Montes and Villalpando-Hernandez8, Reference Mennella and Beauchamp9). In certain ethnic traditions, lactating mothers are encouraged to consume alcoholic beverages as a galactagogue(Reference Flores-Heurta, Hernandez-Montes, Argote and Villalpando10, Reference Walter11) or to sedate their infants(Reference Adams and Davidson12). In such cases, ethanol ingested by breast-feeding mothers is passed to nursing infants through breast milk. Chicken soup flavoured with sesame oil and rice wine (CSSR), which is consumed by Taiwanese (ethnically Chinese) mothers during the traditional Chinese ‘doing-the-month’ ritual, is another example of the mother–milk–infant exposure pathway(Reference Chien, Liu, Huang, Hsu and Chao13). ‘Doing the month’ is a 30 d, culturally defined postpartum period of prescribed behaviours such as eating certain foods deemed beneficial to convalescing mothers. The ingredients in CSSR are believed to benefit new mothers by enhancing energy and protein intake, increasing peripheral blood circulation, and thus facilitating recovery by redressing the ‘hot–cold’ imbalance resulting from pregnancy(Reference Liu-Chiang14).
Apart from the potential health effects imposed directly on breast-fed infants(Reference Mennella and Gerrish15, Reference Little, Anderson, Ervin, Worthington-Roberts and Clarren16), ethanol intake during lactation has been found to alter the metabolism of lipid and other ingredients in the milk of mammals(Reference Heil, Hungund, Zheng, Jen and Subramanian17–Reference Vilaro, Vinas, Remesar and Herrera20), hence changing the composition of breast milk and its nutritional status. Additionally, maternal ethanol intake also inhibits the release of suckling-induced oxytocin and attenuates the milk-ejecting reflex(Reference Subramanian21–Reference Cobo23), likely resulting in less milk production(Reference Mennella24) and less milk consumption by the breast-fed infant(Reference Mennella and Beauchamp9). Although CSSR is typically prescribed during the Taiwanese Tso-Yueh-Tzu (also known as ‘doing-the-month’) ritual, its effects on lactation characteristics have not been investigated.
The present study examined whether the traditional Chinese alcoholic diet (CSSR) affects lactation parameters and causes short-term changes in maternal milk and blood composition.
Methods
Study protocol
The study design followed those of previous studies(Reference Mennella and Beauchamp9, Reference Mennella24). In particular, a within-subject repeated measurement was applied to control for individual variation. Each subject was tested on two days with a 1-week interval and randomly received either CSSR (exposure section) or NASC (non-alcoholic sesame oil-flavoured chicken soup, control section). Each subject was tested approximately 15 d after delivery. The experiment was conducted at a commercial Tso-Yueh-Tzu centre, a maternity health-care setting where subjects reside for about a month after discharge from hospital. Blood and milk samples were obtained from each subject at fixed time points during a 3 h test period after imbibing CSSR or NASC. Various constituents in the samples were determined. The study protocol was reviewed and approved by The Committee on Human Study at Taipei Medical University (Taipei, Taiwan).
Subject selection
The enrolment criterion for the study was that CSSR was part of subjects’ normal diet after delivery. Twenty-three healthy, non-smoking expectant mothers were recruited from the gynaecology and obstetrics clinics at Taipei Medical University Wan-Fang Hospital (Taipei, Taiwan). Informed consent was obtained from each subject prior to the study. Testing commenced approximately 15 d after delivery. Average age, height, weight, BMI and body adipose rate of the subjects was 24·5 (sd 3·4) years, 158·8 (sd 6·5) cm, 62·5 (sd 9·6) kg, 24·6 (sd 2·6) kg/m2 and 37·1 (sd 6·6) %, respectively. Nineteen mothers were primiparous and four were multiparous.
Preparation of stimuli
In the present study, CSSR comprised black sesame oil, de-boned chicken breast, aged ginger and rice wine (alcohol 19·5 %; Taiwan Tobacco and Liquor Co., Taiwan) and was prepared in accordance with commercially available recipes. Based on such preparation method, the alcohol level in CSSR reached approximately 40 mg/ml by continuous boiling for 65 min. Alcohol concentrations in CSSR were stable (variable within ±4 % of the initial level) under freezing (−10°C) for 1 month. The CSSR thus prepared was stored in a freezer in small individual portions (soup liquid only) for experimental use. Since the entire study was conducted over a period of several months, CSSR was prepared on three different occasions, and alcohol levels and other macronutrients were analysed after each preparation. Analysis of CSSR by GC equipped with a flame ionization detector revealed alcohol levels of 40·65 (sd 1·82) mg/ml(Reference Chien, Liu, Huang, Hsu and Chao13). A target alcohol dosage of 0·30 g/kg body weight was achieved by administering to each subject ∼8 ml of soup per kilogram of body weight. As the amounts and frequencies of CSSR consumed during the ‘doing-the-month’ period are subjective, this alcohol level was comparable with that used in other studies and reportedly produced a widely favoured taste. The non-alcoholic chicken soup flavoured with sesame oil (NASC) used as control was prepared using a similar method to that employed in CSSR preparation.
Experimental procedure
Subjects were asked to refrain from alcohol consumption for three days prior to the experiment to ensure a low baseline alcohol level; compliance was confirmed by 3 d dietary records(Reference Mennella and Beauchamp9).
On the morning of the sampling day, subjects (after fasting for 8 h) were weighed, blood samples (10 ml) were taken by in-dwelling catheter and milk from each breast was emptied by electric breast pump. Milk and blood samples collected at this stage were used as baseline levels (time 0). Two servings of a cereal snack (∼630 kJ (∼150 kcal)) were then administered to each subject before CSSR or NASC intake. After 1 h, CSSR or NASC, which was heated in a microwave oven, was consumed by the subjects within 15 min. Subjects were allowed to tend to their infants but were instructed not to breast-feed.
After consumption of CSSR, milk samples (2 ml) were obtained by means of an electric breast pump from each subject at 10, 20, 30, 40, 60 and 90 min, for alcohol analysis. At 120 min after consuming CSSR or NASC, milk was emptied from both breasts (15 min for each breast) by electric breast pump and pooled. Since this procedure required 30 min (15 min for each breast), the mid-point (135 min) was adopted as the sampling time for further analysis. The volume excreted and the time required for ejection of the first milk droplet were recorded. Venous blood samples (2 ml) were obtained using an in-dwelling catheter at 20, 40, 60 and 90 min after intake of CSSR for alcohol analysis. At 150 min after consumption of both soups, venous blood samples (10 ml) were obtained from each subject and analysed for the test constituents. The blood samples were drawn into Vacutainer tubes containing heparin salt(Reference Jones, Jonsson and Kechagias25). Blood and milk samples were centrifuged at 2000 and 4000 rpm, respectively, and the supernatants were then stored at −80°C until further analysis.
Sample analysis
Analysis of macronutrients in soups
The standard methods of the Association of Official Analytical Chemists(26) were used to analyse the macronutrient levels in CSSR and NASC, i.e. water, crude ash, crude protein, crude fat, carbohydrates and energy. Specifically, water and crude ash were determined by a heat evaporation/weighing method; crude protein was analysed using Kjeldahl–H2SO4 titration; crude fat was determined by hexane extraction and a heat evaporation/weighing method; carbohydrates were estimated by subtracting the weights of water, crude ash, crude protein and crude fat from the sample; and energy level was calculated using the formula: energy (kcal) = [crude protein (g)] × 4 + [carbohydrates (g)] × 4 + [crude fat (g)] × 9. The concentrations of these constituents were 83·72 (sd 0·00) g/dl, 0·41 (sd 0·01) g/dl, 1·39 (sd 0·01) g/dl, 1·95 (sd 0·12) g/dl, 1·51 (sd 0·03) g/dl and 120·5 (sd 4·6) kJ/dl (28·8 (sd 1·10) kcal/dl), respectively.
Analysis of blood and milk composition
Composition of blood (serum) was analysed by commercial RIA-based test kits. Specifically, TAG (mg/dl), total protein (mg/dl), glucose (mg/dl), cholesterol (mg/dl), glutamic oxaloacetic transaminase (GOT, U/l) and glutamic pyruvic transaminase (GPT, U/l) were analysed using Kodak Ektachem Clinical Chemistry slides (TRIG Slide, TP Slide, GLU Slide, CHOL Slide, GOT Slide and GPT Slide, respectively) from Eastman Kodak Company (NY, USA); insulin (μU/l) was determined using an immunology analyser (Roche Elecsys System 1010) from F. Hoffmann-La Roche Ltd (Basel, Switzerland); and prolactin (ng/ml) was analysed with a Prolactin MAIAclone kit from Adaltis Italia SpA (Bologna, Italy). Similarly, the commercial assay kits NEFA FA115, Ranbut RB 1007 and Lactate LC 2389 from Randox Laboratories Ltd (Crumlin, UK) were used for determination of NEFA (mmol/l), β-hydroxybutyrate (mmol/l) and lactate (mg/dl), respectively. The analysis was performed by a certified laboratory.
Milk constituents were analysed by commercial test kits and in accordance with methods described elsewhere. Total protein (mg/dl), β-hydroxybutyrate (mmol/l), NEFA (mmol/l) and lactate (mg/dl) were analysed using the commercial assay kits TP 1630, RB 1007, NEFA FA115 and Lactate LC 2389 (Randox Laboratories Ltd), respectively. Lactose and TAG were analysed using the ELISA method modified from Arthur et al.(Reference Arthur, Smith and Hartmann27) and Cox et al.(Reference Cox, Owens and Hartmann28), respectively. Energy level (kcal) was determined on the basis of the equation presented earlier.
Data analysis
Summary statistics are expressed as means and standard deviations. Student’s t test was utilized to compare differences in blood and milk composition between baseline and specific time points post-treatment. The paired t test was applied to test the significance of exposed (CSSR) and control (NASC) groups. A value of P < 0·05 (two-tailed) was considered statistically significant.
Results
Table 1 presents the macronutrient levels in the two soups under evaluation. CSSR had a significantly lower water content (P < 0·05, t test) but significantly higher crude fat and energy levels (P < 0·05, t test) than NASC. The discrepancy in water content was due to the fact that CSSR was made with rice wine (containing 19·5 % alcohol), while NASC used 100 % water in the preparation. The higher crude fat level in CSSR can be explained by the greater amount of fatty acids (originating from the fat of chicken meat) produced in the alcoholic medium. Higher fat and alcohol level in CSSR resulted in higher overall energy level than that in NASC.
Table 1 Macronutrient levels in the alcoholic and non-alcoholic soups
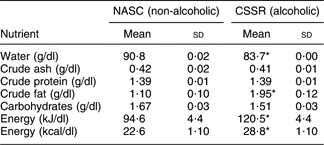
NASC, non-alcoholic sesame oil-flavoured chicken soup; CSSR, chicken soup flavoured with sesame oil and rice wine.
*Mean values were significantly different from those of the control (NASC) group: P < 0·05 (t test).
Table 2 shows mean concentrations of constituents identified in the blood of twenty-three lactating mothers after consuming soup with alcohol dosage of 0·3 g/kg body weight or non-alcoholic control soup. At 150 min after soup consumption, concentrations of TAG, insulin and lactate in blood revealed statistically significant differences (P < 0·05, paired t test) between the CSSR and NASC groups.
Table 2 Mean concentrations of constituents in blood samples of twenty-three lactating women after consuming soup containing 0·3 g alcohol/kg body weight v. non-alcoholic control soup
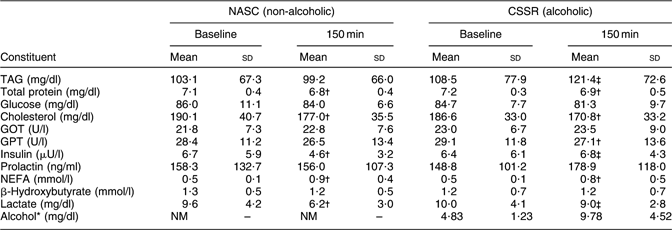
NASC, non-alcoholic sesame oil-flavoured chicken soup; CSSR, chicken soup flavoured with sesame oil and rice wine; GOT, glutamic oxaloacetic transaminase; GPT, glutamic pyruvic transaminase; NM, not measured.
*From Chien et al.(Reference Chien, Liu, Huang, Hsu and Chao13). Note: the lower limit of the reportable range for blood alcohol level was 10 mg/dl.
†Mean values were significantly different from those at baseline: P < 0·05 (paired t test).
‡Mean values were significantly different from those of the control (NASC) group: P < 0·05 (paired t test).
Table 3 displays the mean concentrations of various constituents in the breast milk of twenty-three lactating women after consuming CSSR (alcohol dosage 0·3 g/kg body weight) or NASC. Concentrations of TAG and lactate differed significantly (P < 0·05, paired t test) between the CSSR and NASC groups at the end of the experiment (135 min).
Table 3 Mean concentrations of constituents in breast milkFootnote * of twenty-three lactating mothers after consuming soup containing 0·3 g alcohol/kg body weight v. non-alcoholic control soup
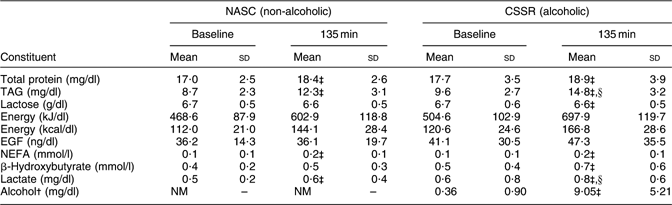
NASC, non-alcoholic sesame oil-flavoured chicken soup; CSSR, chicken soup flavoured with sesame oil and rice wine; EGF, epidermal growth factor; NM, not measured.
* Milk was emptied from both breasts at 120 min post-exposure and pooled. Since this procedure took 30 min (15 min for each breast), the mid-point (135 min) was adopted as the sampling time.
†From Chien et al.(Reference Chien, Liu, Huang, Hsu and Chao13). Note: the detection limit of the method for milk alcohol level was 2·47 mg/dl.
‡ Mean values were significantly different from those at baseline: P < 0·05 (paired t test).
§Mean values were significantly different from those of the control (NASC) group: P < 0·05 (paired t test).
Table 4 presents mean lactation parameters, i.e. the volume excreted and the time required for ejection of the first droplet of breast milk, in the twenty-three lactating women after consuming CSSR or NASC. The results revealed that mothers consuming CSSR required a significantly longer time (P < 0·05) until ejection of the first milk droplet than mothers consuming the non-alcoholic soup (4·4 v. 2·9 min), while the total volume excreted by the CSSR group was less than that excreted by the NASC group (41·3 v. 47·6 ml) but the difference did not reach statistical significance (P > 0·05). Moreover, milk yields were not correlated with alcohol levels in blood or milk (detailed data not shown).
Table 4 Mean lactation parameters of twenty-three lactating women after consuming soup containing 0·3 g alcohol/kg body weight v. non-alcoholic control soup

NASC, non-alcoholic sesame oil-flavoured chicken soup; CSSR, chicken soup flavoured with sesame oil and rice wine.
*At 120 min after consuming CSSR or NASC, milk was emptied from both breasts (15 min for each breast) using an electric breast pump. Volume excreted and time required for the first milk droplet to be ejected were recorded.
†Mean values were significantly different from those of the control (NASC) group: P < 0·05 (paired t test).
Discussion
Influence of alcoholic diet on maternal blood composition
The pharmacokinetics of alcohol in milk and blood after CSSR consumption and its implications have been described in detail elsewhere(Reference Chien, Liu, Huang, Hsu and Chao13). Briefly, milk alcohol peaked at 20–40 min, decreased nearly linearly thereafter, and reached zero level at approximately 175 min post-consumption. Blood alcohol levels followed a similar pattern as in milk but increased to a maximum at 20 min. Large variations in milk (mean 44·4 %) and blood (mean 30·0 %) alcohol level were noted among subjects. At 135 min after consumption of CSSR, the alcohol levels in milk were significantly higher than at baseline (Table 3).
In the current study, mean levels of total protein and cholesterol in blood at 150 min post-consumption of CSSR and NASC were significantly different (lower) from their corresponding baseline levels, while NEFA levels increased compared with baseline (Table 2). Conversely, at 150 min post-consumption of CSSR or NASC, concentrations of TAG, insulin and lactate in maternal blood exhibited statistically significant differences between the two groups (Table 2). These differences can be explained by the following mechanism. The oxidation of ethanol produces acetyl-CoA, which is in turn converted into NEFA and TAG. Meanwhile, lactate in the blood increases in response to the temporally unbalanced oxidation/reduction status in the body resulting from ethanol metabolism(Reference Forsander29).
Influence of alcoholic diet on milk composition
Lactation is a multistage process characterized by increased demand for metabolic substrates to provide the constituents such as lactose, lipid and protein of milk(Reference Heil, Hungund, Zheng, Jen and Subramanian17, Reference Oyama, Couto, Couto, Damaso and Oller do Nascimento30, Reference Kunz, Rodriguez-Palmero, Koletzko and Jensen31). Mean levels of total protein, TAG, NEFA, β-hydroxybutyrate and lactate in milk at 135 min post-consumption of CSSR and NASC differed significantly from their corresponding baseline levels (Table 3), indicating that ingestion of the soups affected milk composition and thus its nutritional status, at least in the short term.
Conversely, mean concentrations of TAG and lactate in milk at 135 min post-consumption (Table 3) revealed statistically significant differences between the two treatments. Such differences were also observed in blood (Table 2), indicating that the milk constituents are a function of blood substrate levels which in turn are affected by diet. However, no significant difference was observed in milk energy levels between the two groups. These findings are compatible with earlier experimental results. For example, ethanol intake during lactation has been found to alter lipid metabolism in rats, as indicated by an increased lipogenesis rate in vivo in the mammary gland and its lipid content(Reference Tavares do Carmo and Nascimento-Cui18–Reference Vilaro, Vinas, Remesar and Herrera20). Rat studies revealed that chronic ethanol ingestion during gestation and lactation also alters mammary gland function, resulting in lower total milk production(Reference Vilaro, Vinas, Remesar and Herrera20). The milk from ethanol-fed rats had increased pH, protein and lipids and decreased lactose compared with controls(Reference Sanchis and Guerri19). This evidence clearly shows that ethanol intake during lactation alters breast milk composition, even though the current findings may be confounded by the higher fat and energy contents in the exposed group (CSSR) than in the control (Table 1). Accordingly, further investigation is needed to address this uncertainty.
Influence of alcoholic diet on lactation performance
Lactation is a hormone-mediated process. Oxytocin is responsible for ejection of milk from the mammary gland, and prolactin promotes milk synthesis(Reference Short32). Numerous studies have found that ethanol blocks oxytocin release and inhibits the milk-ejecting reflex in lactating mothers. For example, an ethanol dose of >1·0 g/kg significantly inhibited milk-ejecting activities (measured via intra-mammary and intra-uterine pressure changes) and the inhibition correlated with administered ethanol dose more closely than with blood ethanol levels(Reference Cobo23). Studies have also demonstrated that suckling-induced oxytocin levels are attenuated following ethanol consumption in non-lactating women(Reference Coiro, Alboni, Gramellini, Cigarini, Bianconi, Pignatti, Volpi and Chiodera22) and lactating rats(Reference Heil, Hungund, Zheng, Jen and Subramanian17–Reference Subramanian21). Similarly, the present study found a statistically significant increase (∼52 %, 4·4 v. 2·9 min, P < 0·05) in the time for ejection of the first milk droplet in the exposed (CSSR) group in comparison with the non-alcoholic diet group. This difference indicates that the milk-ejecting reflex is affected at the dose level of 0·3 g alcohol/kg body weight. A comparable study also identified an increase (∼12 %) in the time until ejection of the first milk droplet after alcohol consumption, although the increase was not statistically significant(Reference Mennella24). Direct inhibition of the central nervous system or impairment of the neurotransmission of infant suckling to the posterior pituitary is the potential mechanism blocking oxytocin release and inhibiting the milk-ejecting reflex(Reference Fuchs33).
Conversely, although prolactin is considered essential for maintaining long-term lactation(Reference Cox, Owens and Hartmann28, Reference Mennella34), previous studies of blood prolactin pharmacokinetics, both in men and non-lactating women, have shown that ethanol consumption generally increases plasma prolactin levels(Reference Volpi, Chiodera, Gramellini, Cigarini, Papadia, Caffarri, Rossi and Coiro35–Reference Earll, Gaunt, Earll and Djuh39). This trend towards increased blood prolactin levels after alcohol exposure was also observed in the current study although the increase was not statistically significant (from 148·8 to 178·9 ng/ml, Table 2). Conversely, animal studies have revealed decreased suckling-induced prolactin excretion after ethanol administration(Reference Subramanian, Chen and Bergeski40, Reference Subramanian and Abel41). Accordingly, no clear temporal correlation can be established between blood prolactin levels and alcohol administration.
Milk yield has also been studied as an indicator of lactation performance. A study of twenty-one mothers demonstrated a statistically significant reduction (∼22 %) in milk yield at 2 h after maternal ingestion of alcohol in juice at 0·3 g/kg(Reference Mennella24). Animal studies further indicated that chronic ethanol consumption reduced milk yields(Reference Sanchis and Guerri19). The present investigation also revealed excretion of less milk (∼15 %, Table 4) at 2 h after CSSR consumption compared with the NASC group; however, the differences were not statistically significant. Such decreases in milk yields in the current study were not found to be correlated with alcohol levels in blood or milk.
As previous studies have demonstrated that milk content can be affected by ethanol intake, the present study accounted for four factors impacting alcohol absorption and pharmacokinetics: (i) alcohol doses were administered based on the subjects’ body weight; (ii) the time alcohol remained in the gastrointestinal tract was controlled by administering two servings of cereal prior to alcohol consumption; (iii) the rate at which alcohol was consumed was controlled as subjects were required to consume their soup within 15 min; and (iv) repeated within-subject measurement was applied to control for intra-individual variation.
Additionally, it is known that the composition of milk is not constant. For example, the fat content exhibits large diurnal variations and increases during the course of each nursing, although it does not vary consistently during lactation(Reference Mitoulas, Kent, Cox, Owens, Sherriff and Hartmann42–Reference Jenness45). Milk lipids are affected by maternal diet(Reference Jensen43, Reference Jensen46); therefore, the randomized repeated measurement design utilized in the current study is beneficial to control for the intra-individual variation potentially resulting from diet. Furthermore, as previous studies have demonstrated that total fatty acid composition remains uniform between 2 and 16 weeks postpartum(Reference Clark, Ferris, Fey, Brown, Hundrieser and Jensen47, Reference Jensen48) and milk TAG remains constant after the first week postpartum(Reference Harzer, Haug and Bindels49), the current finding of significant change in milk TAG after CSSR consumption is less likely to result from maternal diet.
Conclusions
Tso-Yueh-Tzu (‘doing the month’) is an important Chinese cultural practice deemed beneficial for convalescing new mothers. Although contemporary Chinese women question certain taboos in ‘doing the month’ such as refraining from washing the hair for an entire month(Reference Liu-Chiang14, Reference Leung, Arthur and Martinson50), eating prescribed foods such as CSSR remains a crucial practice in the Tso-Yueh-Tzu ritual(Reference Liu-Chiang14, Reference Chan, Nelson, Leung, Cheung and Li51–Reference Whang53). Previous study has demonstrated the potential transfer of alcohol through the mother–milk–infant pathway after consumption of a typical diet, CSSR, during the ‘doing-the-month’ period(Reference Chien, Liu, Huang, Hsu and Chao13). The present investigation further examined whether CSSR consumption affects lactation parameters.
Ingestion of both soups during lactation affected maternal metabolism of certain constituents such as lipids, resulting in changes in levels detected in the blood. Consumption of alcoholic soup influenced TAG, insulin and lactate levels in maternal blood compared with the non-alcoholic preparation. Likewise, ingestion of the soups affected milk composition and its nutritional status, particularly total protein, TAG, NEFA, β-hydroxybutyrate and lactate levels. Intake of alcoholic soup during lactation significantly impacted TAG and lactate levels in the milk. The time until ejection of the first milk droplet was significantly longer in the alcoholic diet group, indicating that at the current dose level (0·3 g alcohol/kg body weight) the milk-ejecting reflex is inhibited. However, blood prolactin, a hormone essential for maintaining long-term lactation level, increased slightly after ethanol intake. Milk yields were affected by the alcoholic soup, but the influence was not statistically significant. Nevertheless, as the current study focused on short-term effects, complementary studies with a longer follow-up would be useful to elucidate the long-term effects of an alcoholic diet on the composition of breast milk and breast-feeding characteristics.
Maternal alcohol ingestion during lactation has no proven benefit to either infants or mothers. Conversely, previous studies have clearly demonstrated that alcohol ingested by lactating mothers can be passed to infants and negatively affect their short-term physiological response and long-term development. Such facts, in conjunction with the current findings that alcohol impacts lactation, suggest that ingestion of alcoholic drinks/foods should be avoided during lactation.
Acknowledgement
Sources of funding: Financial supports for the study came partially from the National Science Council (Taiwan, Republic of China; Grant NSC91-2815-C-241-006-E), Hungkuang Committee of Academic and Research Development (Grant# HKC-89-B-004) and internal funds from Taipei Medical University.
Conflict of interest declaration: None declared.
Authorship responsibilities: Y.-C.C. conceived the study hypothesis, performed data analyses and prepared the manuscript; Y.-J.H. implemented the experiment and collected data; C.-S.H. recruited study subjects and supervised their health status; J.C.-J.C. offered details in human sample collection and analysis; J.-F.L. supervised the study design, data interpretation and implication.
Acknowledgement: Participation of the study subjects is gratefully acknowledged.








