Phyto-oestrogens are polyphenolic, non-steroidal plant-derived compounds, which are structurally related to 17β-oestradiolReference Adlercreutz1. Predominant phyto-oestrogens in the Western diet belong to the subclasses of isoflavones, lignans and prenylflavonoidsReference Fletcher2. Depending on the endogenous oestrogen levels and the target tissue, these compounds can mimic or modulate the activity of circulating oestrogens resulting in potential health effectsReference Ososki and Kennely3. Binding of phyto-oestrogens to the human oestrogen receptors α and β is not the only common feature of this quite diverse group. Their bioavailability is characterized by considerable inter-individual variation, which is partly due to differences in metabolism by the gut microbiotaReference Rowland, Faughnan, Hoey, Wähälä, Williamson and Cassidy4 and the background dietReference Blakesmith, Lyons-Wall, Joannou, Petocz and Samman5, Reference Horner, Kristal, Prunty, Skor, Potter and Lampe6.
Prenylflavonoids including xanthohumol (X), isoxanthohumol (IX) and 8-prenylnaringenin (8-PN), are found in hops (Humulus lupulus L. Cannabaceae) and hop-derived products such as beers and herbal preparations claiming sedative effects, relief of menopausal complaints or breast enhancementReference Coldham and Sauer7, Reference Heyerick, Vervarcke, Depypere, Bracke and De Keukeleire8. Whereas X and IX have no or weak oestrogenic activity, 8-PN is substantially more oestrogenic than other dietary phyto-oestrogensReference Milligan, Kalita, Heyerick, Rong, De Cooman and De Keukeleire9. 8-PN is also unique in respect of its oestrogen receptor specificity, as it binds preferably to oestrogen receptor-αReference Schaefer, Hümpel, Fritzemeier, Bohlmann and Schleuning10.
The weak phyto-oestrogen IX gained much attention recently, since it can be O-demethylated into 8-PN in vitro by the human faecal microbiotaReference Possemiers, Heyerick, Robbens, De Keukeleire and Verstraete11 and by mammalian cytochrome P450 enzymesReference Guo, Nikolic, Chadwick, Pauli and van Breemen12, Reference Nikolic, Li, Chadwick, Grubjesic, Schwab, Metz and van Breemen13. As its concentration in hop-derived products exceeds that of 8-PN by over ten-foldReference Stevens, Taylor and Deinzer14, bioactivation of IX might increase the exposure of man to 8-PN. However, analysis of fifty-one faecal samples revealed large inter-individual variation in the IX conversion capacity of the human intestinal bacteria, which led to a separation of individuals in poor, moderate and high 8-PN producersReference Possemiers, Bolca, Grootaert, Heyerick, Decroos, Dhooge, De Keukeleire, Rabot, Verstraete and Van de Wiele15. Until now, this bioactivation has not been studied extensively in vivo. Schaefer et al. Reference Schaefer, Bohlmann, Schleuning, Schulze-Forster and Hümpel16 detected 8-PN in the urine of two men after a single dose of 10 mg IX mixed into a high-alcoholic beverage. In a small in vivo trial involving three women, Possemiers et al. Reference Possemiers, Bolca, Grootaert, Heyerick, Decroos, Dhooge, De Keukeleire, Rabot, Verstraete and Van de Wiele15 found a clear relationship between the in vitro transformation potential of faecal microbial cultures and the urinary 8-PN excretion after IX consumption.
Understanding the factors influencing the bioactivation of IX in vivo is important to estimate the 8-PN exposure after oral administration of hop-based products. In addition to differences in the composition and activity of the intestinal microbiota, dietary factors may also affect the bioavailability of phyto-oestrogensReference Rowland, Faughnan, Hoey, Wähälä, Williamson and Cassidy4. Macronutrient intake can selectively modify the growth of gut bacteria involved in the bioactivation of phyto-oestrogensReference Rowland, Wiseman, Sanders, Adlercreutz and Bowey17 by changing the intestinal pH, redox potential or transit time or by influencing the availability of substratesReference Cummings and Macfarlane18. In particular, differences in total fat and dietary fibre consumption have been highlighted in previous studiesReference Blakesmith, Lyons-Wall, Joannou, Petocz and Samman5, Reference Horner, Kristal, Prunty, Skor, Potter and Lampe6, Reference Rowland, Wiseman, Sanders, Adlercreutz and Bowey17, Reference Frankenfeld, Patterson, Horner, Neuhouser, Skor, Kalhorn, Howald and Lampe19. The purpose of the present work was: (1) to examine the extent of inter-individual variation in urinary excretion of hop-derived prenylflavonoids; (2) to assess the prevalence of the different 8-PN producer phenotypes; (3) to evaluate the importance of microbial activation of IX towards 8-PN exposure; (4) to identify microbial and dietary factors that are associated with in vivo 8-PN production. Therefore, a dietary intervention trial with fifty healthy post-menopausal Caucasian women was conducted.
Materials and methods
Chemicals and reagents
The isolation of X from spent hops (i.e. the vegetative residue left after liquid or supercritical CO2 extraction of hop cones; NATECO2, Wolnzach, Germany), isomerization of X into IX and preparation of 8-PN were performed as described by Possemiers et al. Reference Possemiers, Heyerick, Robbens, De Keukeleire and Verstraete11. A stock solution of 15 mm-IX in absolute ethanol was prepared. 4-Hydroxybenzophenone (internal standard) was obtained from Fluka Chemie (Buchs, Switzerland). Type H-1 Helix pomatia extract (min. 300 U β-glucuronidase/mg and 15·3 U sulfatase/mg), p-nitrophenol, p-nitrophenyl-β-d-glucopyranoside and p-nitrophenyl-β-d-glucuronide were obtained from Sigma Aldrich (Bornem, Belgium). All solvents were purchased from Biosolve (Valkenswaard, The Netherlands).
Study design and population
An intervention trial with fifty healthy post-menopausal Caucasian women was undertaken to study the microbial metabolism of phyto-oestrogens from hops in vivo and in vitro. Women were eligible for participation if they were not using any exogenous hormone medications, not suffering from a gastrointestinal disease and free of cancers. Women who were receiving an antibiotic therapy (n 2) were scheduled at least 1 month after antibiotic therapy completion. Subject information such as date of birth, weight, height, use of pre-, pro-, syn- or antibiotics, smoking and time of last vaginal bleeding were collected upon recruitment.
Participants consumed their habitual Western-type diets, but were asked to abstain from products based on hops during the trial. A list of prenylflavonoid-containing foods was distributed in order to help the volunteers. After a 4 d washout period, subjects delivered a faecal sample for incubation purposes and microbiological phenotyping, a blank urine sample and two end-expiratory breath samples. Subsequently, three hop-derived dietary supplements per d were administered during five consecutive days and on the fifth treatment day a 24 h urine sample was collected. A self-administered semi-quantitative FFQ was developed to estimate the usual fat, fibre, alcohol, caffeine and theobromine consumption. This FFQ included questions on the average consumption (frequency, daily portion size) of seventy-six food items during the past year and some additional questions involving more detailed information about some product groups. The inclusion of food items was based on knowledge from previously conducted population dietary surveys in Belgium. The validity and reproducibility of this instrument are reported elsewhere (Bolca et al., unpublished data).
The present study was given ethical approval by the Ethics Committee of the Ghent University Hospital (EC UZG 2005/022). The volunteers were fully informed on the aims of the study and gave their written consent.
Sample collection and processing
End-expiratory breath samples were collected using the QuinTron GaSampler system (Ecce Medical, Schoten, Belgium). The alveolar air samples were analysed immediately by GC-Flame ionisation detector (FID). The background room air was found to contain less than 2 ppm methane. Participants were considered to be methane producers when their breath methane concentration exceeded 3 ppmReference Soares, Lederman, Fagundes-Neto and de Morais20.
Volumes of the 24 h urine samples were measured and aliquots were stored at − 20°C. For hydrolysis of conjugated prenylflavonoids, a 33 g/l solution of Type H-1 Helix pomatia extract in sodium acetate buffer (0·1 m, pH 5) was prepared. Unfrozen urine (15 ml) was added to sodium acetate buffer (15 ml) and β-glucuronidase/arylsulfatase solution (30 μl) and the samples were mixed and incubated for 1 h at 37°C. The hydrolysed samples were spiked with 90 μl internal standard (0·4 m-4-hydroxybenzophenone in ethyl acetate) before extraction. The solid-phase extraction Bond Elut® C18 silica columns (5 ml, 500 mg, Varian, St.-Katelijne-Waver, Belgium) were pre-conditioned with 5 ml methanol, 5 ml water and 5 ml sodium acetate buffer (0·1 m, pH 5), consecutively. After sample application, the cartridges were rinsed with 5 ml water and 5 ml methanol/water (2:3) and the compounds of interest were eluted with 2 ml methanol using a VacMaster 20 sample processing unit (IST, Hengoed, Mid Glamorgan, UK). Finally, the solvent was evaporated at room temperature under a gentle stream of N2 and the residue was dissolved in 100 μl methanol, transferred into HPLC vials and stored at − 20°C prior to analysis.
Faecal suspensions were prepared by homogenizing 20 g faeces with 100 ml phosphate buffer (0·5 m, pH 7) supplemented with 1 g/l sodium thioglycolate. Particulate material was removed by centrifugation at 400 g for 2 min. To assess the 8-PN production capacity of each faecal culture, in vitro experiments were set up following the incubation and extraction protocols developed by Possemiers et al. Reference Possemiers, Heyerick, Robbens, De Keukeleire and Verstraete11. Aliquots of faecal suspensions were also stored at − 80°C for measurement of bacterial β-glucosidase and β-glucuronidase activities.
Hop-derived dietary supplements
Two different batches of hop-based capsules (MenoHop®, Biodynamics bvba, Ostend, Belgium), were used: twelve women (24 %) were given BD01 capsules; thirty-eight women (76 %) ingested BD02 capsules. Composition and manufacturing information have been described by Heyerick et al. Reference Heyerick, Vervarcke, Depypere, Bracke and De Keukeleire8. The concentrations of prenylflavonoids were measured by HPLC-UV after extracting 200 mg of the capsules with 10 ml methanol and diluting the supernatant five times in methanol. BD01 contained 0·23 ± 0·01 mg IX, 0·10 ± 0·01 mg 8-PN and 1·38 ± 0·03 mg X per capsule; BD02 contained 1·20 ± 0·04 mg IX, 0·10 ± 0·01 mg 8-PN and 2·04 ± 0·06 mg X per capsule.
Quantification of methane by GC-FID
Alveolar air samples were taken using a Pressure-Lok precision analytical syringe (Alltech Ass., Deerfield, IL, USA). The methane concentrations were measured with a Chrompack CP 9000 GC equipped with a flame ionization detector (Chrompack, Middelburg, The Netherlands), based on the protocol of Boeckx et al. Reference Boeckx, Van Cleemput and Villaralvo21. The analyses were carried out using the following conditions: injection temperature 65°C; oven temperature 35°C; detector temperature 250°C. A mixture of 50·3 (sd 1·5) ppm CH4 in Ar was used as standard gas (L'Air Liquide, Liege, Belgium).
Quantification of prenylflavonoids by HPLC-UV
Quantitative analyses of the prenylflavonoids extracted from the urine samples, incubation media or capsules were performed by HPLC-UV using a Waters 2695 Alliance separations module (Waters, Milford, MA, USA) equipped with a Waters 996 photodiode array detector and Waters Millenium software v3.20 as reported by Possemiers et al. Reference Possemiers, Heyerick, Robbens, De Keukeleire and Verstraete11. Detection was done simultaneously at 295 nm (for IX, 8-PN and 4-hydroxybenzophenone) and at 370 nm (for X). Peaks were identified by comparison of the retention times and UV spectra with those of authentic isolated reference compounds. Concentrations were calculated based on peak area integration of the analytes and the internal standard.
Measurement of faecal enzyme activity
Unfrozen faecal suspensions were centrifuged at 5000 g for 5 min and diluted five times in phosphate buffer (0·5 m, pH 7)Reference Hoey, Rowland, Lloyd, Clarke and Wiseman22. For the assessment of β-glucosidase and β-glucuronidase activities, the suspensions were incubated aerobically for 30 min at 37°C with p-nitrophenyl-β-d-glucopyranoside (2·5 mm) or p-nitrophenyl-β-d-glucuronide (2·5 mm), respectively. Release of p-nitrophenol was recorded with a Tecan Sunrise™ absorbance reader (Tecan Benelux, Mechelen, Belgium) at 405 nm before and after incubation. The absorbance of a series of different concentrations of p-nitrophenol was used to calculate the enzymatic activities, according to the method of Berg et al. Reference Berg, Nord and Wadstrom23.
Statistical approach
All extractions and analyses were performed in triplicate means and sd were calculated. The Statistical Package for the Social Sciences for Windows version 12.0 (SPSS Inc., Chicago, IL, USA) was used to carry out all statistical analyses. Unless reported differently, a P value of 0·05 was used as threshold for significance. Two-sided significance levels are quoted. Tests for normality of the data and equality of the variances were performed using the Kolmogorov–Smirnov and Levene's test, respectively. Comparison of normally distributed data was performed with Student's t test or ANOVA. The non-parametric Mann–Whitney U and Kruskal–Wallis test were used to compare means of non-normally distributed data. Partial Pearson correlation coefficients adjusted for the type of hop treatment were computed to measure associations between urinary parameters.
Subjects were separated into statistically different groups using the TwoStep cluster analysis protocol. Associations between the producer phenotype and subject characteristics, urinary, microbial and dietary parameters were evaluated using nominal logistic regression with poor 8-PN producers as reference category. Cross-classification analysis calculated the agreement between the in vivo and in vitro data.
Results
Subject characteristics and diet
The mean age of the subjects was 57 years with a range from 46 to 74 years. Twenty women (40 %) were classified as overweight (BMI ≥ 25 kg/m2); five (25 %) of these were obese (BMI ≥ 30 kg/m2). Four women (8 %) were smokers. The majority had not used antibiotics during the past year and consumed less than one pre-, pro- or synbiotic preparation per month (Table 1). Forty-nine good-quality FFQ (98 %) were included in the analysis. The average consumption of total fat, SFA, MUFA, PUFA, fibre and alcohol was compared with the guideline daily amounts for seniors proposed by the Belgian Health Council24 (Table 2). Only 10 % of the study population reported a daily fibre intake of at least 30 g/d, as recommended. While the Belgian Health Council advises not to drink any alcohol on a regular basis, the Eurodiet guideline allows up to 12 g/d24. The estimated alcohol intake of thirty-seven participants (76 %) was less than 12 g/d; thirteen (35 %) of these reported to drink less than one alcoholic beverage per month. Twenty-eight subjects (57 %) consumed at least two cups of regular coffee per d (223·82 (sd 61·13) mg caffeine/d), while twenty-one (43 %) consumed not more than one cup (125 ml) of regular coffee per d or drank coffee with reduced caffeine content, decaffeinated or surrogate coffee (45·52 (sd 40·00) mg caffeine/d).
Table 1 Time since last antibiotic therapy and frequency of consumption of pre-, pro- or synbiotic preparations, expressed as a percentage of individuals (n 50)*

* For details of subjects and procedures, see Materials and methods.
Table 2 Actual and guideline daily intakes and percentage of the FFQ reports*† (Mean values and standard deviations for forty-nine subjects)
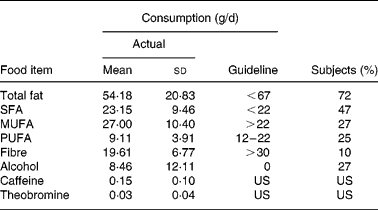
* FFQ reports are in agreement with the recommendations for seniors proposed by the Belgian Health Council24.
† For details of subjects and procedures, see Materials and methods.
US, unspecified.
Pulmonary methane excretion
Thirty-three (66 %) volunteers had methane (4·05–27·95 ppm) in their alveolar air samples and were included in the group of methane producers. The other participants (34 %) did not exceed the selected methane threshold in their breath (1·20–3·00 ppm). Age, BMI, use of pre-, pro-, syn- or antibiotics, smoking and dietary parameters (total fat, SFA, MUFA, PUFA, fibre, alcohol, caffeine and theobromine intake) were comparable for both methane producers and non-producers.
Urinary excretion and recovery of prenylflavonoids
IX, 8-PN and X in the 24 h urine samples were quantified as daily excretion and percentage of the daily doses recovered (Table 3). The inter-individual variations in urinary excretion were eighteen-fold for IX, thirteen-fold for 8-PN and six-fold for X between subjects of the BD01 group; between the subjects of the BD02 group, the inter-individual variations were thirty-fold for IX, forty-fold for 8-PN and twenty-seven-fold for X. A thirteen-fold variation in the renal excretion of total prenylflavonoids was observed in both treatment groups. The recoveries of IX, 8-PN and X were low, especially for X. Less than 1 % of the dosed X was found in the urine. A 1·5-fold increase in X dosage resulted in an equivalent rise in urinary X excretion. In contrast, multiplying the ingested amount of IX with a factor five decreased the recovery of IX significantly (P = 0·005). Although the capsules contained the same amount of 8-PN, the average 8-PN excretion was higher in the BD02 group, but the difference was statistically not significant. When controlling for the type of hop treatment, the urinary recovery of IX correlated modestly with the recovery of 8-PN (R 0·293; P = 0·041). There was a stronger correlation between the recoveries of X and IX (R 0·445; P = 0·001) and of X and 8-PN (R 0·605; P < 0·001), respectively. Neither the faecal β-glucosidase (24·6 ± 19·9 μmol p-nitrophenol/h per g faeces) nor the β-glucuronidase (15·5 (sd 9·9) μmol p-nitrophenol/h per g faeces) activity correlated with the excretion of IX, 8-PN or X.
Table 3 Concentrations of isoxanthohumol (IX), 8-prenylnaringenin (8-PN) and xanthohumol (X) in 24 h urine samples of post-menopausal women who ingested a hop-based food supplement BD01 (n 12) or BD02 (n 38) three times per d for 5 d* (Mean values and standard deviations)
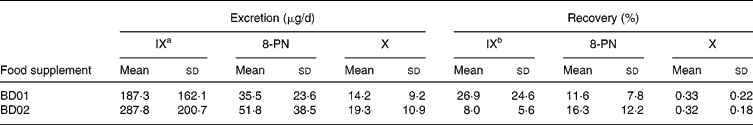
a P = 0·054, BD01 v. BD02.
b P = 0·005, BD01 v. BD02.
* For details of subjects and procedures, see Materials and methods.
Based on the ratio 8-PN:(8-PN + IX) excreted in the urine and the type of hop treatment, three different groups were formed (Fig. 1(A)). Thirty-one (62 %), twelve (24 %) and seven (14 %) women were classified as poor, moderate and strong in vivo 8-PN producers, respectively. The average urinary recovery of 8-PN (P = 0·014) and IX (P = 0·002) were significantly different between these groups, while the average recovery of X (P = 0·374) was similar.
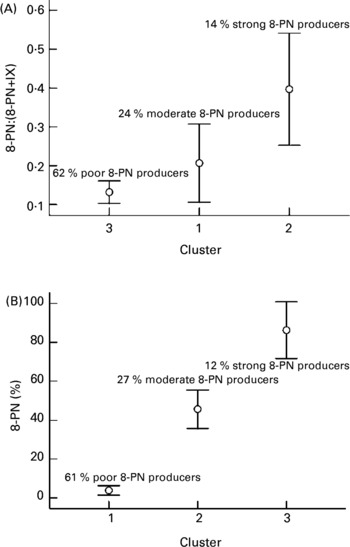
Fig. 1 Clustering of individuals (n 50) in three significantly different groups, namely, poor, moderate and strong 8-prenylnaringenin (8-PN) producers, based on (A) the ratio 8-PN:(8-PN + isoxanthohumol (IX)) excreted in the 24 h urine and the type of hop treatment; (B) the capacity of the faecal microbiota to convert IX in 8-PN. Values are means with their standard deviations represented by vertical bars. For details of subjects and procedures, see Materials and methods.
Microbial metabolism of prenylflavonoids
The microbial metabolism of IX was highly variable ranging from 0 up to 100 % IX transformed into 8-PN and led to a separation of the subjects in poor (61 %), moderate (27 %) and strong (12 %) in vitro 8-PN producers (Fig. 1(B)). These clusters had significantly different average urinary recoveries of 8-PN (P = 0·027) and X (P = 0·032), but not for IX. The percentages IX transformed and 8-PN produced by the faecal cultures correlated inversely (R − 0·650, P < 0·001). The prevalence of poor, moderate and strong in vitro 8-PN producers was not significantly influenced by the type of hop treatment.
Factors associated with 8-prenylnaringenin production
Associations between the in vivo 8-PN producer phenotype and subject characteristics, microbial and dietary parameters are presented in Table 4. Individuals who had not taken antibiotics at least 6 months before participation were more likely to be strong in vivo 8-PN producers (P = 0·035). There was some indication (0·050 < P < 0·075) that pulmonary methane excretion was positively related with in vivo 8-PN production. A lower reported alcohol consumption (P = 0·039) and a higher intake of theobromine (P = 0·050) were associated with the strong in vivo 8-PN producer phenotype.
Table 4 Subject characteristics, microbial and dietary parameters in relation to the in vivo 8-prenylnaringenin (8-PN) producer phenotype, expressed as OR and upper and lower 95 % CI derived from nominal logistic regression with poor in vivo 8-PN producers as reference category*

* For details of subjects and procedures, see Materials and methods.
IX, isoxanthohumol.
Most of the subject characteristics, urinary, microbial and dietary parameters were not strongly associated with the in vitro 8-PN producer phenotype (Table 5). However, positive trends (0·050 < P < 0·075) between urinary excretion and recovery of X and in vitro 8-PN production were observed. Lower estimated alcohol (P = 0·041) and higher theobromine intake (P = 0·047) correlated with the in vitro strong 8-PN producer phenotype.
Table 5 Subject characteristics, urinary, microbial and dietary parameters in relation to the in vitro 8-prenylnaringenin (8-PN) producer phenotype, expressed as OR and upper and lower 95 % CI derived from nominal logistic regression with poor in vitro 8-PN producers as reference category*

* For details of subjects and procedures, see Materials and methods.
IX, isoxanthohumol; X, xanthohumol.
As alcohol consumption differed between the 8-PN producer phenotypes, this topic was examined more thoroughly. Alcoholic beverages were included in four items of the FFQ: aperitif; wine and champagne; beer; liqueurs and spirits. In addition to the frequency and daily portion size of beer consumption, questions on the type of beer (no or low alcoholic, pilsner type or strong beer) were included to estimate the usual intake of both alcohol and prenylflavonoids. Neither in vivo nor in vitro 8-PN production correlated significantly with the usual beer consumption. Twenty-six women (53 %) reported to drink less than 200 ml beer per month, whereas the others had an average estimated beer consumption of 56 (sd 70 ) ml/d. Habitual X, IX, 8-PN and total prenylflavonoid intakes were similar in all 8-PN producer phenotypes and ranged from 0 to 102 μg/d for X, 0 to 630 μg/d for IX, 0 to 21 μg/d for 8-PN and 0 to 753 μg/d for total prenylflavonoids.
Cross-classification analyses for 8-PN producer phenotypes estimated from in vivo and in vitro data indicated that six women (12·2 %) were misclassified, while twenty-six subjects (53·1 %) were classified correctly and forty-three participants (87·7 %) were classified correctly or in the adjacent category.
Discussion
The prenylflavonoids X, IX and 8-PN are found in hops (Humulus lupulus L. Cannabaceae) and hop-derived products, including beers and food supplements. As bioactivation of IX to the potent phyto-oestrogen 8-PN might increase the oestrogenic potency of such products, the metabolism of prenylflavonoids was studied in vivo and in vitro. In order to work with the target population for phyto-oestrogen therapy and to minimize the variations in circulating oestrogen concentrations and their possible interactions with the phyto-oestrogen metabolismReference Frankenfeld, McTiernan and Tworoger25 and liver enzymesReference Pollock, Wylie and Stack26, only healthy hormone-naive women were included. The recoveries of the dosed IX, 8-PN and X were low and considerable inter-individual variations were observed. Classification of the volunteers into poor (60 %), moderate (25 %) and strong (15 %) 8-PN producers based on either urinary excretion or microbial bioactivation capacity of faecal samples gave comparable results. Recent antibiotic therapy seemed to affect the 8-PN production negatively. A positive trend between methane excretion and 8-PN production was observed. A lower alcohol and a higher theobromine intake were associated with the strong 8-PN producer phenotype.
Less than 1 % of the administered X was excreted in the urine and a 1·5-fold increase in X dosage resulted in an equivalent rise in urinary excretion. After oral administration of 20 to 500 mg/kg body weight X as a pure compound or as a hop extract, Avula et al. Reference Avula, Ganzera, Warnick, Feltenstein, Sufka and Khan27 found 0·06–0·49 % of the dosed X in the urine and more than 99·5 % in the faeces of rats. These results suggest that X is poorly absorbed through the intestinal wall, thereby resulting in a low oral bioavailability. This feature may be an important bottleneck in the development of X as a novel broad-spectrum cancer chemopreventive agentReference Gerhaüser, Alt and Heiss28. Based on the apparent permeability coefficients assessed in the Caco-2 cell monolayer model, Nikolic et al. Reference Nikolic, Li, Chadwick and van Breemen29 predicted a rather efficient intestinal absorption via passive diffusion for 8-PN. This rapid enteral absorption was also found after a single oral administration of 8-PN to healthy post-menopausal womenReference Rad, Hümpel, Schoemaker, Schleuning, Cohen and Burggraaf30, as well as in the present study. The recoveries of 8-PN and IX in the 24 h urine samples were comparable to those reported for the isoflavones genistein (8–16 %) and daidzein (15–50 %)Reference Setchell, Brown, Desai, Zimmer-Nechimias, Wolfe, Jakate, Creutzinger and Heubi31–Reference Xu, Wang, Murphy, Cook and Hendrich33.
The observed inter-individual variation in urinary excretion of prenylflavonoids was considerable and higher than the 5·5-fold variation in 24 h renal excretion of 8-PN reported by Schaefer et al. Reference Schaefer, Bohlmann, Schleuning, Schulze-Forster and Hümpel16 in a small trial involving six subjects. In comparison, concentrations of the isoflavones genistein and daidzein in 24 h urine samples varied between subjects twelve- to twenty-four-fold and thirteen to eighty-five-fold, respectivelyReference Blakesmith, Lyons-Wall, Joannou, Petocz and Samman5, Reference Wiseman, Casey, Bowey, Duffy, Davies, Rowland, Lloyd, Murray, Thompson and Clarke32, Reference Karr, Lampe, Huchins and Slavin34. Lampe et al. Reference Lampe, Karr, Hutchins and Slavin35 and Rowland et al. Reference Rowland, Wiseman, Sanders, Adlercreutz and Bowey17 reported a wider range of excretion of the microbial daidzein metabolites equol and O-desmethylangolensin and the enterolignans enterolacton and enterodiol in human subjects.
The urinary recovery data indicated that the excretion of IX and 8-PN was dependent on the ingested amount of IX. A five-fold increase in IX dosage decreased the recovery of IX significantly. Simultaneously, the average 8-PN excretion increased although the ingested amount of 8-PN was unchanged. This is probably due to the conversion of IX into 8-PN in vivo. Urinary excretion of 8-PN after consumption of IX has also been reported by Schaefer et al. Reference Schaefer, Bohlmann, Schleuning, Schulze-Forster and Hümpel16 and Possemiers et al. Reference Possemiers, Bolca, Grootaert, Heyerick, Decroos, Dhooge, De Keukeleire, Rabot, Verstraete and Van de Wiele15, and in both studies inter-individual differences were noticed. In addition, the urinary recovery of 8-PN was significantly different between poor, moderate and strong 8-PN producers. As the prevalence of the different 8-PN producer phenotypes was equivalent in both treatment groups, these results suggest that the extent of the conversion depends on the IX dose administered. Cross-over studies with different IX doses are warranted to investigate this in more detail.
The 8-PN production was estimated from in vivo as well as in vitro data. The study population was separated into poor (62 %), moderate (24 %) and strong (14 %) in vivo 8-PN producers. Based on the microbial bioactivation capacity of the faecal cultures, a similar classification prevailed. This is in quite good agreement with the incubation experiments of Possemiers et al. Reference Possemiers, Bolca, Grootaert, Heyerick, Decroos, Dhooge, De Keukeleire, Rabot, Verstraete and Van de Wiele15, which separated faecal samples into slow (63 %), moderate (21 %) and high (16 %) IX converters. The hypothesis that microbial and dietary factors give rise to these different 8-PN producer phenotypes was tested.
The inverse relationship between use of antibiotics and 8-PN production indicates that intestinal microbiota are involved. Pulmonary methane excretion may serve as an indicator of methane production by intestinal microbiotaReference Bond, Engel and Levitt36. All humans harbour methanogens in the colon, but methane only appears in the breath if the concentration of Methanobrevibacter smithii exceeds 108/g dry weight faecesReference Miller and Wolin37. Age-related increases in transit time and carbohydrate malabsorption promote methanogenesisReference Fernandes, Wolever and Rao38 and may explain the high prevalence of methane producers in the present study. H2 gas is formed in the colon by a variety of hydrolytic and saccharolytic bacteria to dispose reducing equivalents during fermentationReference Calloway, Colasito and Mathews39 and is consumed by methanogenic, homoacetogenic and sulphate-reducing microbiota. Comparison of the prevalence of the different H2-consuming microbiota in faecal samples of poor, moderate and strong 8-PN producers will unravel the relationship between pulmonary methane excretion and 8-PN production.
Several observational studies have reported differences in dietary intake, particularly fat and fibre, in relation to equol and enterolignan production capacitiesReference Blakesmith, Lyons-Wall, Joannou, Petocz and Samman5, Reference Horner, Kristal, Prunty, Skor, Potter and Lampe6, Reference Rowland, Wiseman, Sanders, Adlercreutz and Bowey17, Reference Frankenfeld, Patterson, Horner, Neuhouser, Skor, Kalhorn, Howald and Lampe19. The present study did not reveal significant differences. To our knowledge, the effects of alcohol, caffeine, and theobromine consumption on the gut microbiota and, hence, their phyto-oestrogen metabolism have not been investigated directly. As these compounds are rapidly and almost completely absorbed in the stomach and the small intestine, they are not expected to importantly affect the intestinal bacteria, although caffeine intake has been associated with the microbial metabolism of daidzeinReference Frankenfeld, McTiernan and Tworoger25, Reference Frankenfeld, Atkinson, Thomas, Goode, Gonzalez, Jokela, Wähälä, Schwartz, Li and Lampe40. There is some suggestive evidence for an inhibitory effect of alcohol on the activity of CYP1A2Reference LeMarchand, Franke, Custer, Wilkens and Cooney41, Reference Rizzo, Hispard, Dolbeault, Dally, Leverge and Girre42, the mammalian enzyme system that has been shown to demethylate IX into 8-PN in vitro Reference Guo, Nikolic, Chadwick, Pauli and van Breemen12. Thus, the involvement of hepatic enzymes in the bioactivation of IX as suggested by Schaefer et al. Reference Schaefer, Bohlmann, Schleuning, Schulze-Forster and Hümpel16 may explain why a lower reported alcohol consumption was associated with the likelihood of being a strong in vivo 8-PN producer. CYP1A2 activity accounts for almost 95 % of the demethylation of caffeineReference Higdon and Frei43, but contributes only partially to the elimination of theobromineReference Gates and Miners44. Although low and high caffeine consumers were equally present in the current study population, intake of this CYP1A2 inducer could not be linked to 8-PN production. The mechanism of the positive correlation between theobromine consumption and the strong 8-PN producer phenotype remains unclear. As a constituent of cacao, theobromine is consumed by a large proportion of the population, but it possesses little pharmacological activity. It is important to note that only 10 % of the study population had a daily fibre intake above 30 g, as recommended for seniors by the Belgian Health Council24. As a consequence, the effect of fibre consumption or the fat:fibre ratio on the conversion of IX into 8-PN may have been overlooked in this study. Similarly, the influence of the well-known induction of CYP1A2 activity by tobacco smoking could not be clarified, as only four participants (8 %) were smokers. Even though the present study was not specifically designed to address this question, it is unlikely that prior consumption of hop-derived products influences the ability to convert IX into 8-PN, since no differences in the habitual intake of prenylflavonoids were found between the different 8-PN producer phenotypes. Analogously, soya intervention studies failed to stimulate equol production in low equol producersReference Lampe, Skor, Wähälä, Howald and Chen45, Reference Vedrine, Mathey, Morand, Brandolini, Davicco, Guy, Remesy, Coxam and Manach46.
Cross-classification analyses for 8-PN producer phenotype estimated from in vivo and in vitro data indicated that 12·2 % of the women were misclassified, while 53·1 % were classified correctly. This shows that the in vitro incubation experiments give a good indication of the 8-PN producer phenotype and, additionally, stresses the important contribution of the intestinal microbiota towards the in vivo bioactivation of IX. Although the in vivo bioavailability is the final result of intestinal absorption, human and microbial metabolism, cellular retention, distribution and excretion, these results show the potential of faecal incubations as an appropriate screening assay.
Although biotransformation is generally regarded as a process of metabolic inactivation prior to excretion, there are many well-known examples of metabolic activation. Demethylation by cytochrome P450 enzymes or by gut microbiota represents a common metabolic pathway for activation of pro-oestrogens, such as the isoflavone formononetinReference Batterham, Shutt, Hart, Braden and Tweeddale47, Reference Lundh, Pettersen and Kiessling48 and the synthetic steroid mestranolReference Schmider, Greenblatt, Von Moltke, Karsov, Vena, Friedman and Shader49. Similarly, in vivo O-demethylation of IX into 8-PN increased the oestrogenic potency of the hop-derived food supplements in this study, since inter-individual differences in daily excretion of more than 200 μg 8-PN or 2–20 μg 17β-oestradiol equivalentsReference Milligan, Kalita, Pocock, Heyerick, De Cooman, Rong and De Keukeleire50 were observed.
In summary, we showed that individuals can be phenotyped as poor (60 %), moderate (25 %) or strong (15 %) 8-PN producers based on either urinary excretion data or faecal incubation experiments. This inter-individual variation in 8-PN production could be linked to differences in microbial and dietary factors. From this study, we conclude that the oestrogenic potency of hop-derived products depends on the 8-PN producer phenotype and the concentration of IX.
Acknowledgements
This work was funded by a PhD grant of the Institute for the Promotion of Innovation through Science and Technology in Flanders (IWT-Vlaanderen) to Selin Bolca. Tom Van de Wiele is a postdoctoral fellow of the fund for scientific research – Flanders (Belgium) (FWO-Vlaanderen). We thank Marleen Temmerman and Marleen De Block for help in recruiting participants. We also acknowledge the women who generously volunteered for this trial. Lynn Vanhaecke is gratefully thanked for critically reading the manuscript.










