The pathogenesis of dementia and nutrition
While slowing of cognition and some short-term memory loss occur with normal ageing, there is also an increase in a pathological cognitive condition, dementia, with age(Reference Christensen1, Reference Anstey and Low2). The condition affects globally over 35 million people, and will more than triple in the USA in the next four decades(Reference Querfurth and LaFerla3). Dementia is defined by its symptomatology. Demented patients have chronic functional difficulty with memory, in addition to one of the following abnormalities: deficits in executive function (problem-solving, organising a plan to carry out a multi-step activity), agnosia (naming people or objects), aphasia (perception) or apraxia (executing speech or movement)(Reference Kelley and Petersen4–Reference Riddle6). Those with some cognitive impairment but that does not meet the definition of ‘dementia’ are considered to have ‘mild cognitive impairment’(Reference Kelley and Petersen4).
The most common form of dementia, which occurs in more than half of all cases, is Alzheimer's disease, which is characterised pathologically by amyloid plaques and neurofibrillary tangles(Reference Querfurth and LaFerla3). A significant minority of cases of dementia, having radiological evidence of infarction, can be referred to as having vascular or multi-infarct dementia. However, vascular pathology may also be present in Alzheimer's disease, and the term ‘mixed dementia’ is sometimes referred to patients who have features of both Alzheimer's and vascular dementia(Reference Dickstein, Walsh and Brautigam7). Together, these conditions account for more than 80 % of all cases of dementia. Parkinson's disease and alcohol are the other causes of dementia; and rarer dementias also have their distinctive pathologies.
The pathogenesis of Alzheimer's disease is a complex, much studied, but yet incompletely understood topic, the totality of which cannot be addressed fully in this paper. Abnormal processing of amyloid peptides whose function is not well understood, occurs as a result of both genetic and other factors, in which mitochondrial failure, formation of neurofibrillary tangles involving mutations of the tau protein, and reduction of the number of synapses in the hippocampus are all involved(Reference Querfurth and LaFerla3). Defective epigenetic DNA repair has also been implicated(Reference Mastroeni, McKee and Grover8–Reference Wang, Oelze and Schumacher10).
The effect of vascular disease on the brain may contribute to at least some of these pathological processes. Vascular pathology is present in autopsy specimens of 60–90 % of all patients with Alzheimer's disease(Reference Querfurth and LaFerla3). As previously mentioned, Alzheimer's disease and vascular dementia can coexist.
A proinflammatory state, which is characteristic of the metabolic syndrome (a syndrome defined by central adiposity and two or more of the following: (1) high fasting plasma glucose, (2) low-HDL cholesterol, (3) high blood pressure or (4) high TAG)(Reference Ford11), has also been found to be related to dementia and systemic vascular disease(Reference Frisardi, Solfrizzi and Seripa12, Reference Obunai, Jani and Dangas13). Individual components of the metabolic syndrome – hypertension, hyperlipidaemia, diabetes and high BMI – have also been associated with the incidence of dementia(Reference Frisardi, Solfrizzi and Seripa12). Amyloid precursor protein, a protein whose misprocessing is one of the important neuronal cellular abnormalities observed in Alzheimer's disease, is itself a proinflammatory cytokine that is also present in adipocytes(Reference Frisardi, Solfrizzi and Seripa12). There is a relationship between the production of amyloid precursor protein and other proinflammatory cytokines, such as IL-1β, IL-6 and IL-8(Reference Frisardi, Solfrizzi and Seripa12).
Dietary factors may be related to the development of the metabolic syndrome. Persons who consume a ‘Mediterranean-style diet’, less fat, especially saturated fat, meat, and more fruits and vegetables, are less likely to develop this condition(Reference Rumawas, Meigs and Dwyer14). Both animal and human investigations of the roles of individual components of this diet show that many of its constituents, such as plant polyphenols, may have a beneficial effect on the components of the metabolic syndrome(Reference Abete, Astrup and Martinez15–Reference Cefalu and Brantley20, Reference Sivaprakasapillai, Edirisinghe and Randolph21). Among these, berry polyphenols are under active study as treatments. Grape seed compounds prevent the differentiation of adipocytes in vitro, lower blood pressure in humans and reduce ischaemic damage in animal models of myocardial infarction(Reference Sivaprakasapillai, Edirisinghe and Randolph21–Reference Nassiri-Asl and Hosseinzadeh23). Resveratrol, a polyphenol found in numerous fruits including grapes, is being currently investigated as a treatment for the metabolic syndrome in a clinical trial(24, 25).
Much research has also suggested a relationship between nutrition and dementia. Several epidemiological studies have implied an association between fruits and vegetables and dementia(Reference Gu, Nieves and Stern26, Reference Barberger-Gateau, Raffaitin and Letenneur27). One investigation found a relationship between consumption of a variety of foods: fruits, dark, green leafy and cruciferous vegetables, tomatoes, fish, nuts, poultry and salad dressings, with Alzheimer's disease(Reference Gu, Nieves and Stern26). Closer adherence to a Mediterranean diet was also correlated with a lowered risk of dementia(Reference Liebson28).
Polyphenols and their mechanism of action on cognitive illness
Polyphenols are being considered as a potential treatment or preventive agent for dementia. Polyphenols may have multiple physiological effects that serve to protect the brain from the pathogenic mechanism underlying dementia. One effect may be a systemic anti-inflammatory action (Fig. 1). Polyphenols retard systemic vascular inflammation by reducing the production of adipocyte-generated inflammatory cytokines through the regulation of adipocyte development. More than fifteen polyphenols have been found to regulate the adipocyte lifecycle, causing anti-proliferative changes such as preventing adipocyte maturation, retarding lipid storage and inducing adipocyte apoptosis(Reference Rayalam, Della-Fera and Baile29). The best-studied fruit polyphenol, resveratrol, interacts at the cellular level with a gene called Sirtuin 1 (SIRT1)(Reference Borra, Smith and Denu30). Activation of SIRT1 heightens insulin release and sensitivity, and promotes the differentiation of many types of cells, but arrests the development of adipocytes(Reference Cacciapuoti31).
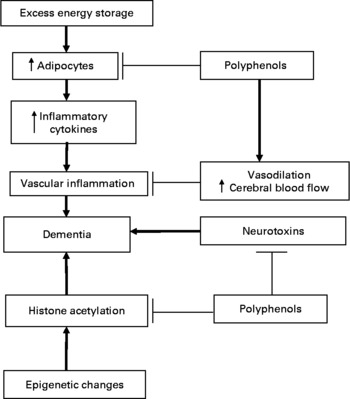
Fig. 1 Potential mechanisms for polyphenol activity against dementing illness.
Several polyphenols, including resveratrol and quercetin, lower inflammatory cytokine levels, improve insulin sensitivity and glucose and insulin levels, and reduce blood pressure in animal models and preliminary human trials(Reference Cherniack32). In one investigation, mice that ingested a high-fructose diet and high-cholesterol diet with added resveratrol had a heightened glucose tolerance, insulin sensitivity and lower cholesterol than mice that did not consume resveratrol(Reference Deng and Hung33). In another study on mice, rodents that ingested resveratrol had lower amounts of visceral fat, and reduced levels of glucose, TAG, cholesterol, and the inflammatory cytokines monocyte chemotactic protein-1 (MCP-1) and TNF-α(Reference Kim, Jin and Choi34). Rats consuming resveratrol for 4 weeks had decreased levels of lipids and serum glucose(Reference Rocha, Souza and Ebaid35). In a study, ten overweight older subjects (average age of 72 years) with reduced glucose tolerance who ingested resveratrol for 4 weeks augmented their insulin sensitivity(Reference Crandall and Oram36).
Quercetin is another polyphenol with anti-inflammatory activity. The polyphenol reduces the output of the proinflammatory cytokines IL-8 and TNF-α in vitro (Reference Boots, Haenen and Bast37). Individuals with the metabolic syndrome who consumed quercetin achieved small reductions in blood pressure(Reference Egert, Bosy-Westphal and Seiberl38).
Polyphenols may be beneficial in the treatment of cognitive disorders by protecting the brain vasculature against the proinflammatory state induced by the metabolic syndrome. Diabetic rats that received resveratrol showed a significantly better performance on memory tests (rats were placed in a device where they learned to avoid foot shocks) than those that received a placebo(Reference Schmatz, Mazzanti and Spanevello39).
Polyphenols, especially cocoa flavonols, may also preserve cognition by causing other beneficial changes in the cerebral vasculature (Fig. 1). These include enhancing cerebral blood flow, local endothelial repair mechanisms, and retarding platelet aggregation and augmenting vasodilation through increasing nitric oxide levels(Reference Williams and Spencer40). Resveratrol promotes the production of nitrous oxide(Reference Cacciapuoti31). This preservation of the cerebral vasculature may allow the maintenance of hippocampal neurons, the loss of which may be an important feature in dementing illness(Reference Williams and Spencer40).
In addition, polyphenols may have direct neuroprotective effects on neurons. Resveratrol averts neuronal loss in several animal models in which neurons are exposed to toxic agents.
Resveratrol protected mouse neurons in vitro when exposed to toxins(Reference Sakata, Zhuang and Kwansa41). In newborn rats, resveratrol reduced neuronal loss after traumatic brain injury(Reference Sonmez, Sonmez and Erbil42). Rats with dementia caused through injections of streptozocin had improved memory and learning (tested by maze negotiation and avoidance of foot shocks) after being given resveratrol(Reference Sharma and Gupta43). In another rat dementia model, in which rats were injected with colchicine, resveratrol again alleviated the deficit in cognitive function measured by the water maze test(Reference Kumar, Naidu and Seghal44). Elderly rats fed pterostilbene, another polyphenol found in grapes and blueberries. had better performances on the water maze test than those fed a control substance, and pterostilbene provided even greater in vitro protection in a chemical-induced neurotoxicity model than resveratrol(Reference Cherniack32).
While the mechanism of this neuroprotective effect has not been fully elucidated, there are many possible hypotheses. Polyphenols activate the neurotransmitter on neurons that facilitate neuronal and microglial growth gene pathways(Reference Williams and Spencer40). A polyphenol subtype, flavonoids, which include resveratrol, quercertin and epigallocathechin gallate, in animal models modulate the synthesis, processing and disposal of amyloid peptides that may be important in the pathogenesis of dementia(Reference Williams and Spencer40).
Polyphenols may induce epigenetic changes that are neuroprotective (Fig. 1). Histone acetylation may be implicated in the pathogenesis of several dementing illnesses, including Alzheimer's disease and Parkinson's disease(Reference Stilling and Fischer45, Reference Iraola-Guzman, Estivill and Rabionet46). SIRT1, which may be a target of resveratrol action, also codes for an enzyme known as histone deacetylase(Reference Huber, McBurney and Distefano47). This enzyme causes conformational changes in histones about which DNA is wrapped, that reveal the DNA to prepare it for transcription by RNA polymerases(Reference Sweatt48). Mice engineered with a defect in histone acetylation show greater memory deficits in old age(Reference Peleg, Sananbenesi and Zovoilis49).
Clinical trials on the use of resveratrol in combination with other substances in the treatment of dementia are now underway. At the Bronx, New York, United States Department of Veterans Affairs Medical Center, an investigation is underway in which sixty subjects with Alzheimer's disease are being given grape juice supplemented with resveratrol, malate and glucose, or a placebo drink for 1 year(50). At the Medical College of Wisconsin, another placebo-controlled trial is taking place in which fifty subjects with Alzheimer's disease are taking one 215 mg tablet a day for 1 year of Longevinex, a dietary supplement that contains resveratrol, quercetin (another grape polyphenol), rice bran, ferulic acid and 1200 IU of vitamin D3 (51).
Fruit juices and extracts
Polyphenols occur naturally in fruits and plants, which can be synthesised into fruit juices and extracts. The polyphenols in these compounds may act individually or synergistically to protect cognition through the same mechanisms as do individual polyphenols.
Consumption of fruit extracts and juices may preserve neurons and improve cognition in aged rodents(Reference Shukitt-Hale, Lau and Joseph52, Reference Joseph, Shukitt-Hale and Willis53). Blueberry products are among the most well-studied in this context. Blueberry supplements increase the levels of neuroprotective heat shock proteins in aged rats(Reference Galli, Bielinski and Szprengiel54). In a study, 6-month-old (young) rats that were fed blueberry extracts for 8 months (until old age) had better performances on a water maze test (a test of recall and spatial learning in which rats are placed in water and given cues to find a hidden platform to allow it to leave the water)(Reference Joseph, Fisher and Cheng55) than those fed a placebo(Reference Joseph, Shukitt-Hale and Denisova56). Addition of a blueberry supplement to 15-month-old (elderly) rats significantly enhanced their ability on an object recognition test(Reference Goyarzu, Malin and Lau57). In another trial, old rats that ate a blueberry extract for 2 months had significantly better balanced walking across a wire or rotating rod and negotiated the water maze more quickly than at baseline, which control rats fed a placebo supplement did not(Reference Joseph, Shukitt-Hale and Denisova58). However, in a similar trial in which the rats were given a blueberry supplement for an extra month, the rodents demonstrated improved motor function as demonstrated by the ability to hang on to a tilted screen, but did not have improved cognitive function in water maze tests(Reference Shukitt-Hale, Galli and Meterko59). Rats that had cognitive impairment induced by injection of a neurotoxic agent (kainic acid) and consumed over 2 months a blueberry extract had a better maze performance than those that did not(Reference Duffy, Spangler and Devan60). When transgenic mice engineered to create an Alzheimer's disease model of dementia were given a blueberry supplement, they maintained better coordination, strength, muscle tone and balance, but did not have fewer amyloid plaques in their brains(Reference Joseph, Denisova and Arendash61). Blueberry extracts also preserved neurogenesis in the hippocampus of elderly rats(Reference Casadesus, Shukitt-Hale and Stellwagen62).
An investigation of the benefit of blueberry juice in the treatment of people with cognitive impairment has been published(Reference Krikorian, Shidler and Nash63). A total of nine subjects, mean age 76·2, drank 6–9 ml/kg of commercially manufactured blueberry juice a day for 3 months. In the study, seven other subjects consumed a placebo drink. At the end of 3 months, those who consumed blueberry juice had a 41 % improvement on the Verbal Paired Associate Learning Test (a test of the associations between two unrelated words, 13·2 v. 9·3 on a 0-to-20 word pair scoring scale; P = 0·009) and a 33 % improvement on the California Verbal Learning Test (a test of list learning and recall, 9·6 v. 7·2 on a 0-to-16 word scoring scale; P = 0·04). In addition, on the Verbal Paired Associate Learning Test, those who consumed blueberry juice had 86 % higher scores as compared to those who consumed the placebo drink (13 v. 7; P = 0·03), but there was not a significant difference between groups in scores on the California Verbal Learning Test.
Grape products are also being actively investigated. Grape seed extract has been observed to increase antioxidant enzyme levels in rodent brains(Reference Devi, Jolitha and Ishii64). Mixtures of grape polyphenols inhibit the formation of amyloid plaques in mouse dementia models(Reference Ono, Condron and Ho65, Reference Wang, Ho and Zhao66). Young mice genetically engineered to become demented that consumed a grape polyphenol extract performed better on the water maze test than those given water instead(Reference Wang, Ho and Zhao66).
The first published human trial showing the benefit of a grape-derived product on cognition was recently published. In this trial, twelve subjects with mild cognitive impairment drank 6–9 ml/kg of Concord grape juice or a placebo for 3 months(Reference Krikorian, Nash and Shidler67). Subjects who drank grape juice had a 20 % improvement in their score (38 v. 33 items) on a 44-item California Verbal Learning Test (P = 0·04) from baseline.
At the University of Oslo, Norway, a trial has recently been completed in which elderly individuals who are not demented but had a ‘gradual subjective memory decline’ were given 9 weeks of a 50 % bilberry (European blueberry), 50 % red grape juice mixture in a double-blinded, placebo-controlled trial(68). The results of the trial have not yet appeared in print.
Other fruit juices have improved cognition in animal models, and some have been used in human trials. In a previously mentioned trial which included a blueberry extract, a strawberry supplement also enhanced rats' speed at completing a water maze and ability to cross a wire(Reference Joseph, Shukitt-Hale and Denisova58). In another trial involving blueberry juice, a cranberry extract enhanced the ability of rats to cling to a wire, but did not affect cognition(Reference Shukitt-Hale, Galli and Meterko59). In a study, fifty normal elderly individuals drank 32 ounces a day of a drink containing 27 % cranberry juice or a placebo(Reference Crews, Harrison and Griffin69). However, there were no differences in neuropsychological testing between subjects in the two groups at the end of this 6-week study. Elderly rats that drank blackberry juice completed the water maze test in less time and were able to balance on a rotating rod for longer than those given a placebo(Reference Shukitt-Hale, Cheng and Joseph70). Old rats that drank plum juice for 2 months negotiated the water maze more quickly, but those that consumed a plum powder had no benefit(Reference Shukitt-Hale, Kalt and Carey71). Apple juice prevented cognitive impairment and increased amyloid-β in aged mice caused by dietary folate or apoE deficiency(Reference Tchantchou, Chan and Kifle72–Reference Chan and Shea74). In an open-label trial, twenty-one elderly persons with Alzheimer's disease drank two four-ounce glasses of apple juice daily for 30 d. The institutional caregivers of these subjects described a 27 % improvement in behavioural symptoms, although there was no change in cognition(Reference Remington, Chan and Lepore75).
Future considerations
Thus far, the research on the use of fruit polyphenols in the treatment of cognitive impairment appears promising. However, there are many further considerations that need to be addressed in the design of more definitive studies.
First, one important consideration is the optimum age for the initiation of therapy. The pathogenic progress resulting in dementia, which might be the consequence of a systemic inflammatory process on the vasculature, might occur years before the onset of symptoms. If this is the case, the optimum time for the initiation of therapy might be in young or middle age, long before symptoms occur. In rodent trials, starting treatments in middle age, which might occur after more than a year in a 2-year lifespan, is not a great difficulty. However, in a human trial, one might have to give the supplement for decades to observe the optimum effect, making the study unfeasible.
In addition, the bioavailability of polyphenols consumed from supplements has been called into question(Reference Scalbert and Williamson76–Reference Rossi, Mazzitelli and Arciello78). The bioavailability of most polyphenols, especially those of higher molecular weight is quite limited(Reference Scalbert and Williamson76, Reference Bravo77, Reference Del Rio, Borges and Crozier79–Reference Cottart, Nivet-Antoine and Laguillier-Morizot82). They are poorly absorbed and extensively metabolised first in the intestine, where they are conjugated through glucuronidation, methylation or sulphation(Reference Manach, Williamson and Morand81). They are then transported to the liver, where they undergo further metabolism(Reference Manach, Williamson and Morand81). Finally, they are readily excreted in the urine or in the bile, resulting in low serum levels for most polyphenols(Reference Del Rio, Borges and Crozier79, Reference Manach, Williamson and Morand81, Reference Cottart, Nivet-Antoine and Laguillier-Morizot82). Pharmacokinetic studies in humans have confirmed this pattern for resveratrol and quercetin, which have been analysed both in single- and multiple-dose administration. However, many of the investigation of the bioavailability of other polyphenols have relied upon the ingestion of single doses of substances. There is controversy involving the accuracy of the assays used to detect the levels of polyphenols and their metabolites, resulting in additional uncertainty about possible error(Reference Del Rio, Borges and Crozier79, Reference Crozier, Del Rio and Clifford80). Studies of resveratrol pharmacokinetics may have underestimated serum resveratrol plasma levels due to technical problems in quantification(Reference Cottart, Nivet-Antoine and Laguillier-Morizot82).
Furthermore, it is unclear how readily the small quantities that enter the blood stream cross the blood–brain barrier(Reference Rossi, Mazzitelli and Arciello78). If the effect of polyphenols on the brain is primarily mediated through its systemic effect on the vasculature, the latter concern is less important. Furthermore, the concern about bioavailability is based on studies of the pharmacokinetics of certain individual polyphenols, particularly resveratrol, which may be less applicable for other polyphenols and mixtures. In fact, the effects of polyphenols might be mediated through the active metabolites of ingested polyphenols, or through the synergism of multiple polyphenols in a compound, such as that occurring in fruit extracts or juices(Reference Scalbert and Williamson76, Reference Cottart, Nivet-Antoine and Laguillier-Morizot82).
A related consideration is the form in which polyphenols are consumed. Polyphenols may be given naturally as a fruit, processed into juices, extracts and tablets, or given as individual polyphenols. At present, there is not much evidence about which form is the best to administer.
In a previously mentioned study on the effect of plum extract on rat cognition, the greater effect caused by plum juice than plum powder was postulated to be either the result of a greater polyphenol content of the juice, changes in the form, differences in the amounts and bioavailability of different polyphenols, or caused by differences in the manufacture and storing of juice and powder from the fruit(Reference Shukitt-Hale, Kalt and Carey71). Further chemical and pharmacokinetic studies may be necessary to fully resolve these issues.
Acknowledgements
The concept, preparation and writing of this paper were entirely the work of its sole author. There are no conflicts of interest in the publication of this review, nor was any specific grant from any funded agency in the public, commercial or not-for-profit sector used to prepare it.





