The B vitamin, folate, and its synthetic form, folic acid, have a number of beneficial effects for both women of reproductive age and the general population. The most well-established benefit of folic acid supplementation is a reduction in the primary incidence( Reference Czeizel and Dudas 1 ) and recurrence( 2 ) of neural tube defects (NTD). A dose–response relationship exists between red blood cell (RBC) folate level and NTD risk, with women having RBC folate level of ≥906 nmol/l having the lowest risk( Reference Daly, Kirke and Molloy 3 ). Possible reproductive benefits at birth include a decrease in the prevalence of severe congenital heart defects( Reference Ionescu-Ittu, Marelli and Mackie 4 ), a reduction in the risk of cleft lip( Reference Wilcox, Lie and Solvoll 5 ) and a reduction in the incidence of preterm birth( Reference Bukowski, Malone and Porter 6 ). Folic acid may also reduce the risk of incident hypertension( Reference Forman, Rimm and Stampfer 7 ), improve cognitive function( Reference Durga, van Boxtel and Schouten 8 ) and decrease depression scores in adults( Reference Taylor, Carney and Geddes 9 , Reference Taylor, Carney and Goodwin 10 ). Few of these additional potential benefits have been conclusively established( Reference Malouf and Grimley Evans 11 , Reference Zhang, Willett and Selhub 12 ). A potential risk is masking of vitamin B12 deficiency with long-term neurological sequelae( 13 ). Folic acid may protect against colorectal( Reference Kennedy, Stern and Moretti 14 , Reference Stevens, McCullough and Sun 15 ) and breast( Reference Zhang, Willett and Selhub 12 , Reference Zhang, Cook and Albert 16 ) cancer, but a procarcinogenic effect is postulated in individuals with presence of neoplastic cells and high folate intake. Systematic reviews recommend ongoing surveillance and longer-term studies including high-dose intake( Reference Baggott, Oster and Tamura 17 , Reference Mason 18 ).
Since 1992, in an effort to decrease the incidence of NTD, the US Preventive Services Task Force has recommended a daily supplement of at least 400 μg of folic acid for all women of reproductive age, commencing folic acid supplementation at least 1 month before conception and continuing with daily supplements through the first 2–3 months of pregnancy( 19 ). Furthermore, since 1998, the US Food and Drug Administration has mandated fortification of grain products, flour and pasta (140 μg/100 g cereal product, in order to increase folic acid intake in the general population by 100 μg/d)( 20 ). The American Congress of Obstetricians and Gynecologists also recommends folic acid supplementation for all women of reproductive age, particularly during the periconceptional period( 21 ), a time when many women may rely on oral contraception for pregnancy prevention. Similarly, the Healthy People 2010 Objective in place at the time the present study was conducted was that 80 % of women of reproductive age consume 400 μg of folic acid from fortified foods and supplements daily( 22 ); this was revised in the Healthy People 2020 Objective to a more attainable 26 %( 23 ). Additional objectives are that 33 % take multivitamins or folic acid daily for a month prior to pregnancy and that only 22 % have low RBC folate concentration (<195 ng/ml or 441·9 nmol/l)( 23 ). Despite these recommendations, national surveys indicate that, at most, only 40 % of women of reproductive age take supplements containing folic acid on a daily basis( 24 ). Although nationwide blood folate levels have increased compared with pre-fortification levels, a substantial proportion of women of childbearing potential, including those consuming folate-fortified foods or taking folate supplements, have an RBC folate level below 906 nmol/l( Reference Brown, Jacobs and Hartman 25 – Reference Shuaibi, House and Sevenhuysen 29 ), the level associated with the highest NTD protection( Reference Daly, Kirke and Molloy 3 ).
Folate intake can be assessed using dietary measures such as food recall diaries and FFQ( Reference Buzzard 30 ). Blood folate markers include plasma folate, a measure of recent intake, and RBC folate, which reflects long-term folate stores in the RBC. Correlations between FFQ and plasma folate levels in women range from 0·186 to 0·63( Reference Zhang, Willett and Selhub 12 , Reference Jacques, Sulsky and Sadowski 31 – Reference Yoshino, Nishide and Sankai 38 ), and from 0·00 to 0·28 between FFQ and RBC folate values( Reference van de Rest, Durga and Verhoef 34 , Reference Verkleij-Hagoort, de Vries and Stegers 35 ). These FFQ request information on the consumption of eighty-four( Reference Johansson, Van Guelpen and Hultdin 32 ) to 145( Reference Yen, Zoumas-Morse and Pakiz 37 ) food items for up to 1 year of recall( Reference Zhang, Willett and Selhub 12 , Reference Jacques, Sulsky and Sadowski 31 , Reference Johansson, Van Guelpen and Hultdin 32 ). Previous studies comparing FFQ with blood folate markers have not focused on women of reproductive age electing contraception.
Worldwide, women of reproductive age often choose oral contraceptives (OC) for pregnancy prevention between pregnancies( Reference Jones, Mosher and Daniels 39 , 40 ). Several circumstances, however, may lead to pregnancies that do not benefit from adequate preconceptional folic acid intake: (i) OC misuse( Reference Hou, Hurwitz and Kavanagh 41 ); (ii) premature OC discontinuation with a switch to a less effective method( Reference Huber, Hogue and Stein 42 , Reference Rosenberg and Waugh 43 ); (iii) rapid return to fertility after OC discontinuation( Reference Cronin, Schellschmidt and Dinger 44 ); and (iv) seeking pregnancy without a visit to a clinician( Reference von Stenglin, Buchwald and Lynen 45 ). Given the sustained RBC folate levels seen up to 5 months after discontinuation of a similar OC( Reference Diefenbach, Trummer and Ebert 46 ), folate-containing OC could serve as a novel means of providing preconception folate should a pregnancy occur. As part of a study evaluating the effect of a novel OC fortified with folate on the change from baseline in plasma and RBC folate levels and homocysteine level( Reference Bart, Marr and Diefenbach 47 ), we sought to assess the adequacy of folate status, using both dietary recall and blood folate markers, in a population of women in the USA – where food is fortified – desiring contraception. Furthermore, we investigated the relationship between folate intake measured using the Short Folate Food Frequency Questionnaire (SFFFQ) with blood folate levels.
Methods
Study design and participants
The current study was part of a multi-centre, randomised, double-blind, controlled contraceptive trial (ClinicalTrials.gov identifier: NCT00468481) that enrolled women from eight sites in the USA( Reference Bart, Marr and Diefenbach 47 ). The trial was conducted in accordance with the International Conference on Harmonization/Good Clinical Practice and the principles stipulated by the Declaration of Helsinki. Each site obtained institutional review board approval, and written informed consent was obtained from all women prior to study entry. Study inclusion criteria included healthy women aged 18–40 years requesting contraception with no contraindications for OC use. The study population was designed to be representative of the US population; therefore, we included women of different ethnicities in rural and urban settings. We targeted a racially diverse study population in order to assess whether such differences lead to variations in folate-rich dietary choices. Study sites were divided into East (Maryland, North Carolina, South Carolina, Tennessee and New York) and West (Washington and California). During screening, study site personnel recorded subject-reported racial/ethnic groups from the categories: Caucasian, African American, Hispanic, Asian and Other.
Participants received either: (i) an OC containing 20 μg ethinylestradiol and 3 mg drospirenone for 24 d followed by 4 d of placebo (YAZ®; Bayer HealthCare Pharmaceuticals, Berlin, Germany) and served as controls; or (ii) the same OC fortified with 451 μg levomefolate calcium (Metafolin®) (BEYAZ®; Bayer HealthCare Pharmaceuticals) for 28 d and represented the intervention group (folate-fortified OC). Unlike folic acid, levomefolate calcium does not need to be metabolised to be biologically active and is, therefore, independent of folate-converting enzymes which control the activation of folic acid. Furthermore, levomefolate calcium has less potential than folic acid to mask vitamin B12 deficiency symptoms( Reference Pietrzik, Bailey and Shane 48 ). At equimolar doses, levomefolate calcium and folic acid (451 μg levomefolate calcium is equivalent to 400 μg folic acid) have also been shown to be similarly effective at increasing plasma and RBC folate levels in women of childbearing potential( Reference Lamers, Prinz-Langenohl and Bramswig 49 – Reference Venn, Green and Moser 51 ). To ensure blinding, both medications had identical packaging. Randomisation was allocated in a 3:1 ratio (fortified OC:control) and study sites received treatment group assignment via an interactive voice response system.
Participants underwent the following visits: two screening visits, a baseline visit, six study visits over the course of 24 weeks, and a final visit at 26–28 weeks. We assessed dietary intake at the baseline and final visits for participants using the SFFFQ. Plasma and RBC folate samples were collected at the pretreatment and week 24 visits. The per protocol (PP) participants reported at least 75 % study medication compliance per cycle, had no major protocol deviations and completed 24 weeks of treatment.
The Short Folate Food Frequency Questionnaire
The SFFFQ (© Columbia University 2007) is similar in design to widely used FFQ( Reference Rimm, Giovannucci and Stampfer 52 ). However, it assesses only folate-rich foods consumed in the last week and, therefore, contains fewer items and takes less time to complete( Reference Castaño and Westhoff 53 ). It was developed to serve as a rapid self-administered assessment of dietary folate status in dietary folate equivalents (DFE), units that account for the differences in bioavailability of natural food folate and synthetic folic acid( 13 ). The SFFFQ is used to collect information about vitamin and supplement intake and the number of servings of multiple preparations of thirty-nine folate-containing foods consumed in the past week. Where available, we obtained μg of DFE for food preparations from the US Department of Agriculture's National Nutrient Database for Standard Reference( 54 ); for others, we utilised product labelling or contacted the manufacturer. For fortified foods, we did not record separately the individual contribution to total μg of DFE from natural food folate and synthetic folic acid. For vitamins and supplements, we multiplied standard values of folic acid in μg (400 μg and 800 μg, respectively) by 1·7 to obtain μg of DFE. For individuals who specified other supplements that contain folic acid, we obtained folic acid values from product labelling prior to multiplying by the conversion factor. For the fortified OC, we calculated μg of DFE by multiplying 400 μg by week 24 compliance and by the folate conversion factor. We then calculated daily consumption for diet plus vitamins/supplements plus the contribution from the fortified OC. We assigned participants consuming at least 320 μg DFE/d to meeting the Estimated Average Requirement (EAR), a dietary reference intake measure of adequate dietary intake for a group( 13 ). As we did not collect separately the folic acid contribution (in μg) in fortified foods, we were unable to calculate the Healthy People 2020 Objective regarding ideal intake of folic acid( 23 ).
Only 0·35 % of SFFFQ items had an unspecified number of servings or were missing brand names. We imputed 27 % of the missing values based on individual intake from another visit with comparable intake and the remaining 73 % from the median for all participants who consumed that item if no comparable visit data were available.
Blood assessments
We obtained all blood samples after a reported 12 h period of fasting. Baseline values reflect the mean of the three pretreatment values. Samples for RBC and plasma folate were drawn in separate lithium-heparin coated tubes. RBC folate samples were prepared by dilution with fresh 1 % w/v ascorbic acid solution, followed by centrifugation and incubation in the dark for 30 min before storing at −80°C. Plasma folate samples were centrifuged and stored at −80°C. Specimens were shipped to the reference laboratory, TNO Quality of Life, The Netherlands, for analysis. Plasma and whole-blood folate concentrations were determined using a validated microbiological assay. Each plate included a WHO folate standard control. RBC folate concentrations were calculated using the equation( Reference Bart, Marr and Diefenbach 47 ): {(whole blood folate × 100) − [plasma folate × (100 – haematocrit)]}/haematocrit.
We assigned participants with RBC folate level of ≥906 nmol/l to having optimal folate status for NTD protection( Reference Daly, Kirke and Molloy 3 ). Similarly, participants with RBC folate >441·9 nmol/l met the Healthy People 2020 Objective regarding low RBC folate concentration( 23 ).
Statistical analysis
We performed univariate analyses of demographic and dietary and blood folate data, bivariate analyses (Student's t test for comparison of means and Pearson's χ 2 test for comparison of proportions) to determine associations, paired t tests to determine differences across visits, and calculated Pearson correlation coefficients to compare dietary and blood folate values. We performed the analyses using the statistical software packages SPSS version 18·0 for Windows and SAS version 9·1 for Windows.
Results
Participants were enrolled from 30 April 2007 to 6 September 2008. Overall, 385 participants were randomised, of whom 379 (98 %) received the intervention (either the OC or the folate-fortified OC), 273 (71 %) completed the study and 262 (68 %) completed the study per protocol (Fig. 1). PP participants reported 100 % OC compliance at week 24 (range 89–104 %). One participant at baseline and four participants at the week 24 visit did not complete the SFFFQ. Due to a sample processing error at two clinical sites, valid RBC folate values were not attainable for all participants; valid RBC folate samples were balanced across treatment groups. RBC folate values were obtained for 196 (75 %) participants at baseline and 185 (71 %) at the final visit; 169 (62 %) participants had RBC folate values at both visits. We obtained plasma folate values for all participants at both time points.
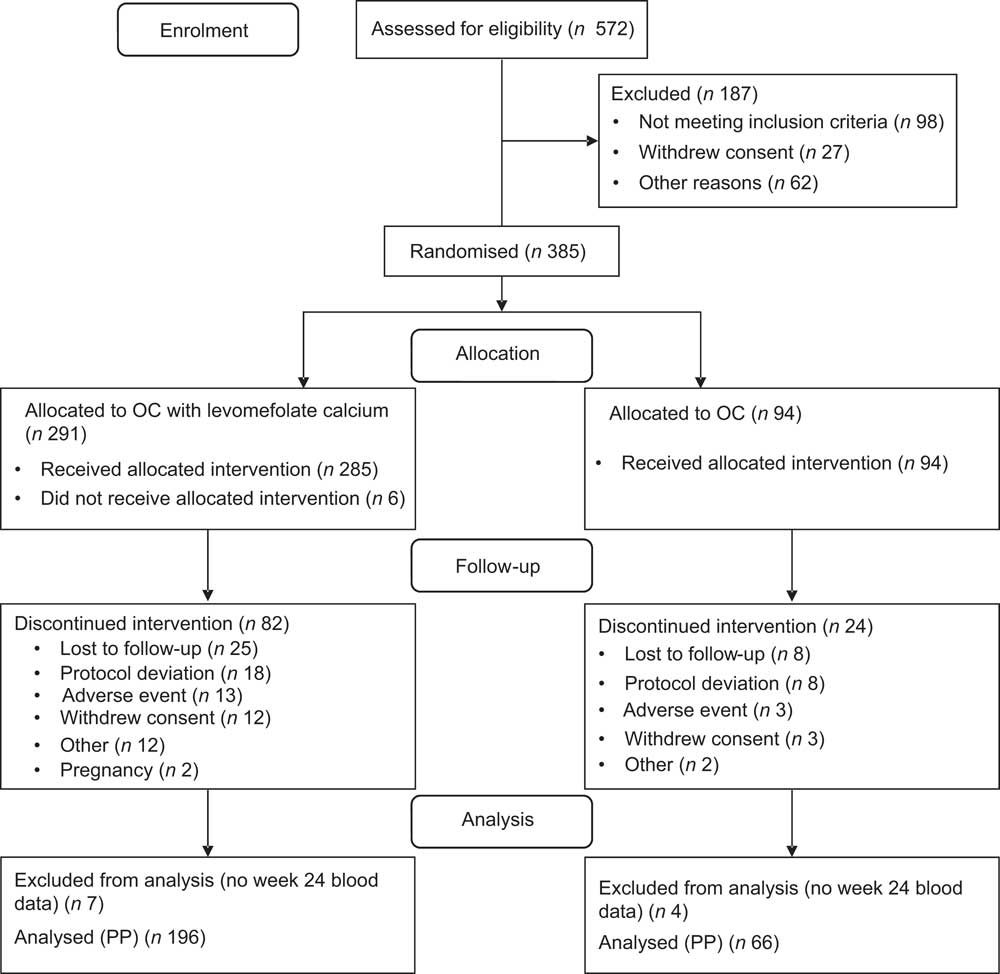
Fig. 1 Participant flow through the study (OC, oral contraceptive; PP, per protocol)
Demographic and baseline participant characteristics are outlined in Table 1. PP participants did not differ from the group excluded from analyses in mean age, region or BMI. Dietary and blood folate values were also similar. There were racial/ethnic differences, however, with fewer PP participants identifying as African American (9 % v. 18 %) and more as Hispanic (14 % v. 9 %) and Asian (10 % v. 4 %; P = 0·03).
Table 1 Demographic and baseline characteristics (per protocol set; n 262): healthy women of reproductive age enrolled in a randomised trial of a folate-fortified oral contraceptive, eight centres in the USA, 2007–2009

Dietary folate equivalents
We calculated daily μg of DFE consumption by visit (Table 2). At baseline, 159 (61 %) participants reported ready-to-eat cereal consumption and their folic acid intake from this dietary component was 225·7 (sd 190·9) μg DFE/d. Only 67 (26 %) reported vitamin or supplement intake and their folic acid intake from supplements was 421·2 (sd 239·8) μg DFE/d. The total DFE intake at baseline was 529·8 (sd 342·1) μg/d.
Table 2 Mean daily folate intakes (in μg of dietary folate equivalents) by study visit: healthy women of reproductive age enrolled in a randomised trial of a folate-fortified oral contraceptive, eight centres in the USA, 2007–2009

OC, oral contraceptive.
Mean values were significantly different from those at baseline: *P < 0·001.
Participants were similar at follow-up for ready-to-eat cereal consumption (n 156, 60 %) and vitamin/supplement intake (n 63, 24 %). The total DFE intake was 534·9 (sd 349·8) μg/d but increased to 1040·3 (sd 471·8) μg/d when the contribution from the fortified OC was included.
Dietary intake of folate did not differ between the groups at baseline (P = 0·6) and at week 24 (P = 0·4) or within the groups between these points in time (controls: P = 0·8, fortified OC: P = 0·7). There was no difference between mean μg of DFE/d at the baseline visit and final visit by folate consumption category (data not shown). Furthermore, there were no differences in the change in total intake mean μg of DFE/d from baseline to follow-up by age category, race/ethnicity, geographic region, study group assignment and BMI category (data not shown).
The folate intake categories that contributed the most DFE to the total consumption at baseline and follow-up were ready-to-eat cereals (26 % and 25 %, respectively) and vitamins/supplements (20 % and 23 %, respectively). Breads (mostly white), pasta, rice, beans (mostly pinto) and romaine lettuce were the five next most common contributors to dietary folate at both visits (all <9 %).
Blood folate levels
Plasma folate values increased during the study for the combined study population (P < 0·001; Table 3); this increase was due to the increase in the fortified OC group. There was no increase in the mean plasma folate values in the control group but there was an increase in the intervention group (P < 0·001). Similarly, RBC folate values increased during the study in the two groups combined (P < 0·001); again, this increase was due to the fortified OC group (P < 0·001). No increase in RBC folate was observed in the control group.
Table 3 Mean blood folate levels (nmol/l) by study visit: healthy women of reproductive age enrolled in a randomised trial of a folate-fortified oral contraceptive, eight centres in the USA, 2007–2009
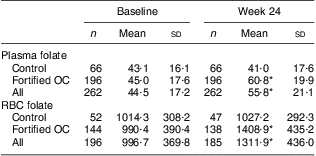
OC, oral contraceptive; RBC, red blood cell.
Mean values were significantly different from those at baseline: *P < 0·001.
Subgroup analyses revealed no difference in both mean plasma and RBC folate levels over the course of the study in women aged over 35 years and for race/ethnicity ‘Other’. Since these subgroups were small (twelve and ten individuals, respectively), this may be a chance occurrence. Similarly, mean plasma folate did not increase over time in the ten women who were underweight.
Folate benchmarks
The percentage meeting or exceeding the EAR (320 μg of DFE/d) in this population was 66 % at baseline (Table 4). Meeting the EAR did not differ by any of the demographic variables assessed. Meeting the EAR increased to 90 % at follow-up (P < 0·001). This was due to the increase in the fortified OC group. Participants meeting the EAR at follow-up tended to be older (P = 0·02). There were no other differences in meeting the EAR at follow-up by any of the demographic variables.
Table 4 Participants meeting folate benchmarks by study visit: healthy women of reproductive age enrolled in a randomised trial of a folate-fortified oral contraceptive, eight centres in the USA, 2007–2009
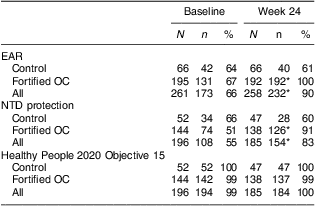
N, total number of participants in the group; n, number of participants in the group meeting the benchmark; EAR, Estimated Average Requirement = 320 μg of dietary folate equivalents/d; OC, oral contraceptive; NTD protection, participants meeting maximum neural tube defect protection threshold of RBC folate ≥906 nmol/l; Healthy People 2020 Maternal, Infant, and Child Health Objective 15 = participants with RBC folate >195 ng/ml (441·9 nmol/l).
Significantly different from baseline: *P < 0·001.
At baseline, only 55 % of participants met or exceeded 906 nmol/l, the value of RBC folate associated with maximum NTD risk reduction. After 24 weeks, however, 83 % overall (91 % in the fortified OC group and 60 % in the control group) met this value (P < 0·001). We found low RBC folate in two participants at baseline and one at follow-up.
Correlations
Total dietary intake in μg of DFE/d correlated with both plasma and RBC folate at baseline (r = 0·34 and r = 0·35, respectively) and remained correlated at follow-up (r = 0·43 and r = 0·41, respectively; Table 5). When evaluating demographic variables for week 24 (data not shown), correlation of dietary intake measured by the SFFFQ with plasma folate was highest in those women who were of a younger age (r = 0·47), lived in the West (r = 0·53) and had a normal BMI (r = 0·48). For RBC folate, the highest correlation was in women who were older (r = 0·52), Caucasian (r = 0·43) and overweight and obese (r = 0·52 and r = 0·43, respectively). There was more variability in correlations at the final visit than at baseline (Fig. 2).
Table 5 Correlations between blood folate levels and dietary folate as assessed via the SFFFQ by study visit: healthy women of reproductive age enrolled in a randomised trial of a folate-fortified oral contraceptive, eight centres in the USA, 2007–2009

SFFFQ, Short Folate Food Frequency Questionnaire; r, Pearson correlation coefficient; RBC, red blood cell.
All correlations were significant at P < 0·05, ρ ≠ 0.
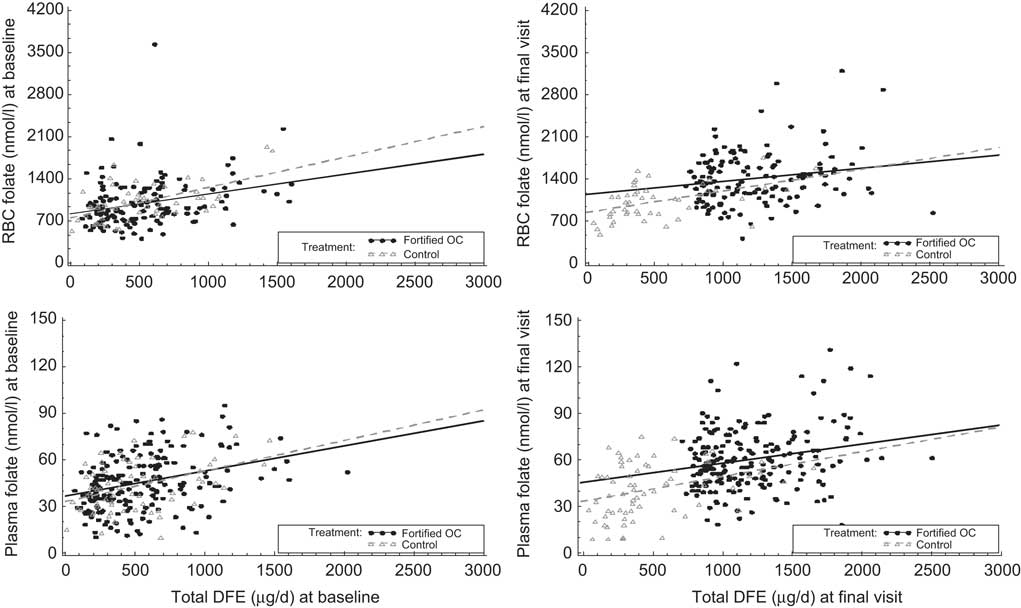
Fig. 2 Correlations between red blood cell (RBC) folate, plasma folate and total dietary folate equivalents (DFE) at baseline and final visit: healthy women of reproductive age enrolled in a randomised trial of a folate-fortified oral contraceptive, eight centres in the USA, 2007–2009
Discussion
In a racially and geographically diverse group of US women exposed to fortified foods who elected to use oral contraception, mean daily dietary intake of folate calculated from the SFFFQ in DFE remained stable over the 6-month study period. Dietary folate levels increased in the group assigned to an OC fortified with levomefolate calcium. Similarly, while plasma and RBC folate levels remained stable in the control group, those in the fortified OC group had an increase in these short- and long-term blood folate stores. These increases improved performance of this population on two folate benchmarks we assessed: meeting the EAR and the NTD protection threshold.
The current study enrolled only women of reproductive age, the age group that stands to benefit the most from adequate folate stores. This is also the age group that often selects an OC as their form of contraception( Reference Jones, Mosher and Daniels 39 ). Unfortunately, OC discontinuation is high( Reference Castano, Bynum and Andres 55 – Reference Peipert, Zhao and Allsworth 57 ), often occurs without consulting a physician( Reference von Stenglin, Buchwald and Lynen 45 ) and leaves women at risk for an unintended pregnancy. Therefore, attaining adequate folate stores in this population is very important. The percentage of participants meeting the EAR measure of group folate intake adequacy was low (66 %) when assessed using the SFFFQ at baseline, but 100 % in the folate-fortified group at 6 months. The percentage not meeting the EAR at baseline was higher than in an analysis of data from the National Health and Nutrition Examination Survey( Reference Bailey, Dodd and Gahche 58 ). This may reflect differences in methodology of dietary assessment between these two studies.
We demonstrated that fortified OC use in a food-fortified population increased the proportion of participants with RBC folate ≥906 nmol/l from half (51 %) to almost all (91 %), thus decreasing their risk of a pregnancy affected by an NTD. Folate stores above baseline would be expected to persist for several months after OC discontinuation( Reference Diefenbach, Trummer and Ebert 46 ). A woman discontinuing such an OC and becoming pregnant within a few months would likely receive the protective reproductive benefits of folate use. A folate-fortified OC could thus serve as a proxy for a daily folic acid supplement and help a woman meet the Healthy People 2020 Objective of daily supplement for a month prior to pregnancy( 23 ).
This population met the Healthy People 2020 Objective for reducing the proportion of women with low RBC folate concentrations at baseline( 23 ). The high RBC folate concentration in our population at both time points may reflect the effect of fortification and supplement intake.
While we did not intend to validate the folate-specific SFFFQ in our study, it performed well in this population. It correlated with plasma and RBC folate levels as well as, if not better than, other FFQ in the existing literature with fewer items and shorter recall requirement. The correlation between the dietary assessment and blood values was positive and highest in the fortified OC group.
The study had some limitations. The SFFFQ relies on self-reported consumption of selected folate-rich foods and assumes unbiased reporting of intake. We collected a single SFFFQ at each time point and thus could not adjust for within-person variability. The SFFFQ collects a week's worth of intake data, however, and dietary intake remained stable throughout the study period. We relied on a national database and, infrequently, product labels for μg of DFE. Databases and product labels can underestimate real folate values because of limitation of analytical methods( 13 , Reference Rader, Weaver and Angyal 59 ) and product labels are not regulated; however, neither of these should have differed by group assignment. In converting to μg of DFE, we assumed fortificants/supplements were consumed with food and not fasting (1·7 conversion factor). This could be an underestimate if supplementary folate was consumed while fasting (2·0 conversion factor).
Our data set included only total folate intake in DFE. For fortified foods it did not collect separately the folic acid and natural food folate contributions. Our food folate benchmark calculations were thus limited to the EAR and we were unable to directly calculate the Healthy People 2020 Objective 14 of 26 % with an intake of at least 400 μg of folic acid/d from fortified foods or supplements. Given that 26 % of the study population reported supplement use at baseline, we can infer that this population likely met this benchmark. Lastly, our results may not be generalisable to countries with different (or no) food fortification or with food consumption that is not captured by the SFFFQ.
The strengths of the study include a geographically and demographically diverse study population of US women of reproductive age. Further strengths are that, as would be expected for diet, reported folate consumption, excluding the fortified OC, was stable during the course of the study. Furthermore, based on diet alone, meeting the EAR did not differ from the beginning to the end of the study. Dietary findings are strengthened by correlation with blood values.
These results underscore the importance of food fortification for achieving adequate DFE. Fortified foods contributed the most to dietary intake; vitamins also contributed a large proportion of DFE. However, neither diet, including fortified foods, nor routine supplement intake alone was sufficient for all women to meet the EAR despite nationwide attempts to increase awareness and intake of folate. Worldwide, folic acid health campaigns have similarly increased knowledge and awareness with moderate increases in consumption( Reference Rofail, Colligs and Abetz 60 ). Ongoing lack of adequate folate intake from dietary sources or supplements alone suggests the need for novel ways of providing supplementation to ensure adequate folate levels. The folate-containing OC tested here provides an example of supplementation targeted at a population (women of reproductive age) that does not always have adequate levels with their current diet and supplementation, and which is the intended beneficiary of improvement in folate status. Furthermore, the SFFFQ performed well in this study population and should be considered when a rapid assessment of dietary folate status is needed.
Acknowledgements
Sources of funding: The study was funded by Bayer HealthCare Pharmaceuticals, Berlin, Germany, the manufacturer of YAZ® and BEYAZ® (the folate-fortified OC). Ethics: The approving ethics committee was Independent Investigational Review Board, Plantation, FL, USA. Conflicts of interest: C.S.-L. and R.L. are employees of Bayer HealthCare Pharmaceuticals Inc. A.A. was an employee of Bayer HealthCare Pharmaceuticals Inc. during the study. P.M.C. has received payments for serving as a scientific advisor for Bayer HealthCare Pharmaceuticals Inc. and as a clinical investigator for Bayer Schering Pharma AG; she also serves as a preceptor for Conceptus and Merck. Authors’ contributions: All authors made substantial contributions to the concept and design or analysis and interpretation of data, and to the drafting of the manuscript or revising it critically for important intellectual content. C.S.-L., A.A. and R.L. contributed to the data review/analysis, data interpretation and review/revision of the manuscript. P.M.C. contributed to the data analysis and interpretation, and drafted and revised the manuscript. In addition, all authors provided final approval of the manuscript. Acknowledgements: The authors would like to thank Amy Evans of inScience Communications, Springer Healthcare, for undertaking an edit of the manuscript.











