- EPIC
European Prospective Investigation into Cancer and Nutrition
- HRT
hormone-replacement therapy
- IGF
insulin-like growth factor
- IGFBP
IGF-binding proteins
- RR
relative risk
The prevalence of obesity has increased substantially over previous decades in most industrialized countries, and a further increase is expected in the future(Reference Seidell1). According to estimates by the International Association for the Study of Obesity provided in April 2007 approximately 40–50% of men and 25–35% of women in the EU were overweight (defined as a BMI between 25·0 and 29·9 kg/m2), and an additional 15–25% of men and 15–25% of women were obese (BMI ≥30·0 kg/m2)(2). Similarly, in 2004 approximately 34·1% of the US population were overweight and about 32·2% were obese(Reference Ogden, Carroll, Curtin, McDowell, Tabak and Flegal3). Obesity is a risk factor for several chronic diseases, most notably hypertension, type 2 diabetes, dyslipidaemia and CHD. Accumulating evidence suggests that obesity is also a risk factor for certain types of cancer. Based on a systematic review of the literature, an expert panel convened by the International Agency for Research on Cancer as part of the WHO concluded in 2002–3 that sufficient evidence exists for a link between obesity and increased risk of colon cancer, post-menopausal breast cancer, endometrial cancer, renal cell cancer and adenocarcinoma of the oesophagus(4, 5). Subsequently, the findings have been published of additional studies that have examined the relationship between excess body fat and cancer risk more extensively. These studies include those that have examined the association between body shape as well as weight gain and cancer risk, and also studies using biomarkers to better define the obesity phenotype that is relevant for cancer risk. Based on the International Agency for Research on Cancer review(4, 5) and on subsequent relevant studies published in the field the present article provides an overview of the association between excess body fat and risk of cancer and of the potential underlying pathophysiology.
Definition and assessment of obesity
The definition of obesity is based on BMI, which is body weight (kg):height2 (m2)(6). BMI is highly correlated with fat mass and morbidity and mortality, and therefore reflects obesity-related disease risk in a wide range of populations. However, there are some important limitations. First, for the same BMI older adults tend to have a higher body fat composition, and therefore risk assessment using BMI is less accurate in these individuals (>65 years of age)(Reference Rimm, Stampfer, Giovannucci, Ascherio, Spiegelman, Colditz and Willett7). Second, current BMI cut-off points for overweight and obesity are suggested to be too high for Asian populations(Reference Choo8). Third, and probably most important, the BMI does not assess body fat distribution. It is well-known that abdominal (central, visceral, android) obesity, which is usually observed in men, is associated with a higher morbidity than the gluteofemoral (peripheral, gynoid) obesity typically observed in women(6). Body fat distribution can most easily be assessed by measurement of the waist and hip circumferences. Current guidelines suggest a waist circumference of 102 cm in men and 88 cm in women, or a waist:hip ratio of 0·95 in men and 0·80 in women, as being the cut-off points for abdominal obesity that are purportedly associated with an increased risk of morbidity(6). Waist circumference shows a close correlation with the amount of visceral adipose tissue, and the latter has been shown to be metabolically more active and to secrete far greater amounts of cytokines and hormones compared with subcutaneous adipose tissue(Reference Pouliot, Despres, Lemieux, Moorjani, Bouchard, Tremblay, Nadeau and Lupien9–Reference Berg and Scherer11). Further, a higher influx of portal fatty acids, cytokines and hormones into the liver from omental adipose tissue may specifically distort hepatic metabolism, including abnormal lipoprotein synthesis, hepatic insulin resistance and increased gluconeogenesis(Reference Eckel, Grundy and Zimmet12, Reference Haslam and James13). Recent large studies have indicated that measurement of waist circumference or waist:hip ratio may be a better disease risk predictor than BMI(Reference Wang, Rimm, Stampfer, Willett and Hu14, Reference Yusuf, Hawken, Ounpuu, Bautista, Franzosi and Commerford15), and intensive research is still ongoing as to which variable(s) are better predictors of disease risk.
Several different diagnostic tools are available to assess body fat composition, such as measurement of (subcutaneous) skinfold by means of a caliper or ultrasound, bioelectrical impedance analysis, densitometry or imaging procedures (computerized tomography, NMR); however, most of these procedures are not readily available in clinical practice, and do not add substantial information for risk assessment in an individual beyond that of BMI and waist circumference(Reference Heymsfield, Allison, Wang, Baumgartner, Ross, Bray, Bouchard and James16).
Obesity and cancers of the colon and rectum
For 2006 it was estimated that 217 400 men and 195 400 women were newly diagnosed with colo-rectal cancer within Europe, accounting for 12·8 and 13·1% of the total cancer incidence in men and women respectively(Reference Ferlay, Autier, Boniol, Heanue, Colombet and Boyle17). In the same year 107 600 men and 99 900 women died of colo-rectal cancer, accounting for 11·3 and 13·3% of all cancer deaths in men and women respectively(Reference Ferlay, Autier, Boniol, Heanue, Colombet and Boyle17). A possible association between obesity and risk of colo-rectal cancer has been examined in many epidemiological studies(Reference Graham, Marshall, Haughey, Mittelman, Swanson, Zielezny, Byers, Wilkinson and West18–Reference Chute, Willett, Colditz, Stampfer, Baron, Rosner and Speizer47) and the International Agency for Research on Cancer and WHO have concluded in their 2002–3 report that there is sufficient evidence that overweight and obesity increases the risk of colo-rectal cancer(5). However, although in most studies body weight and BMI have been found to be positively related to risk of colon cancer in men, weaker or no associations have been reported for women(Reference Graham, Marshall, Haughey, Mittelman, Swanson, Zielezny, Byers, Wilkinson and West18–Reference Chute, Willett, Colditz, Stampfer, Baron, Rosner and Speizer47). Further, among the studies that have examined associations with rectal cancer, most have found no association with body weight or BMI(Reference Gerhardsson de Verdier, Hagman, Steineck, Rieger and Norell19–Reference Le Marchand, Wilkens, Kolonel, Hankin and Lyu22, Reference Chyou, Nomura and Stemmermann29, Reference Le Marchand, Wilkens and Mi34, Reference Phillips and Snowdon44, Reference Chute, Willett, Colditz, Stampfer, Baron, Rosner and Speizer47). The reasons for the apparent discrepancy in the association between body weight and colon cancer risk between men and women have long been unclear, and it has been suggested that one potential reason is that men and women have different body compositions. Fat makes up a lower percentage of the body mass of men (approximately 20%) than of women (approximately 30%). The relationship between body weight and fat distribution also differs between men and women. Higher body weight is more closely related to abdominal obesity than gluteofemoral obesity in men and more closely related to gluteofemoral obesity than to abdominal obesity in women. Furthermore, abdominal obesity has been shown to be more strongly associated with metabolic abnormalities than gluteofemoral obesity(Reference Krotkiewski, Bjorntorp, Sjostrom and Smith48, Reference Lonnqvist, Thorne, Large and Arner49). Hence, assuming that it is primarily visceral adipose tissue and not non-visceral adipose tissue that is involved in tumourigenic processes, body weight and BMI may not accurately reflect the colon cancer risk that is associated with abdominal fat accumulation, at least in women. This hypothesis has recently been supported by findings from the European Prospective Investigation into Cancer and Nutrition (EPIC) that have indicated that abdominal obesity (as defined by waist circumference or waist:hip ratio) is an equally-strong risk factor for colon cancer in men and women, whereas body weight and BMI are associated with colon cancer risk in men but not in women (Fig. 1)(Reference Pischon, Lahmann and Boeing50). Thus, men and women in the highest gender-specific quintile of waist:hip ratio compared with the lowest quintile were found to have a 50% higher risk of developing colon cancer over a mean follow-up period of 6 years(Reference Pischon, Lahmann and Boeing50). Further support for the hypothesis that abdominal obesity rather than total obesity is an equally-strong risk factor for colon cancer in men and women comes from the observation within EPIC that plasma levels of C-peptide (a marker of pancreatic insulin secretion that is tightly correlated with visceral fat accumulation) have been shown to be similarly strongly related to risk of colon cancer in men and women(Reference Jenab, Riboli and Cleveland51). By contrast, within EPIC neither the anthropometric measures nor plasma C-peptide levels were significantly related to rectal cancer(Reference Pischon, Lahmann and Boeing50, Reference Jenab, Riboli and Cleveland51), suggesting that neither total obesity nor abdominal obesity substantially influences the risk of this type of cancer.

Fig. 1. Relative risk of colon cancer according to quintiles of BMI in men (A) and women (B), and relative risk of colon cancer according to quintiles of waist:hip ratio in men (C) and women (D) during a mean follow-up of 6·1 years of 368 277 participants from the European Prospective Investigation into Cancer and Nutrition(Reference Pischon, Lahmann and Boeing50). Relative risks for BMI were adjusted for age, study centre, smoking status, education, alcohol intake, physical activity, fibre intake and consumption of red and processed meat, fish and shellfish and fruit and vegetables. Relative risks for waist:hip ratio were additionally adjusted for height. Values are means and 95% CI represented by vertical bars. For BMI P=0·006 and P=0·40 for trend in men and women respectively and for waist:hip ratio P=0·006 and P=0·002 for trend in men and women respectively.
The pathophysiology underlying the association between abdominal obesity and increased colon cancer risk is unclear. Some authors have suggested that components of the metabolic syndrome, particularly insulin resistance and subsequent hyperinsulinaemia, may be the underlying link, which may reflect the growth-promoting effects of insulin(Reference Giovannucci52–Reference McKeown-Eyssen54). These speculations are also supported by studies that have found that subjects with type 2 diabetes are at increased risk of colon cancer(Reference Hu, Manson, Liu, Hunter, Colditz, Michels, Speizer and Giovannucci55, Reference Seow, Yuan, Koh, Lee and Yu56) and by studies that have found positive associations between plasma insulin and C-peptide levels and risk of colon cancer (see earlier discussion)(Reference Giovannucci53, Reference Ma, Giovannucci, Pollak, Leavitt, Tao, Gaziano and Stampfer57, Reference Wei, Ma, Pollak, Rifai, Fuchs, Hankinson and Giovannucci58). Insulin is known to have growth effects as well as metabolic effects, and data from a variety of sources suggest that insulin is functionally involved in colo-rectal carcinogenesis(Reference Koenuma, Yamori and Tsuruo59, Reference Wu, Fan, Masui, Rosen and Mendelsohn60). Hyperinsulinaemia is also related to increased levels of bioavailable insulin-like growth factor (IGF)-1, which is known to have cancer-promoting effects(Reference Aaronson61–Reference Wu, Yakar, Zhao, Hennighausen and LeRoith64). Insulin interacts with the IGF-1 axis by reducing the synthesis of IGF-1-binding proteins (IGFBP), therefore increasing the bioavailability of IGF-1(Reference Kaaks, Lukanova and Kurzer65). Experimental and observational studies suggest that IGF-1 may be involved in the development of colo-rectal cancer(Reference Giovannucci53, Reference Wei, Ma, Pollak, Rifai, Fuchs, Hankinson and Giovannucci58, Reference Sandhu, Dunger and Giovannucci63, Reference Calle and Kaaks66–Reference Gunter and Leitzmann69). More recent data suggest that adipose tissue-derived cytokines and hormones, collectively termed adipokines, may also be involved in tumourigenesis, including leptin, which stimulates growth of colonic epithelial cells(Reference Aaronson61–Reference Wei, Ma, Pollak, Rifai, Fuchs, Hankinson and Giovannucci63, Reference Stattin, Palmqvist, Soderberg, Biessy, Ardnor, Hallmans, Kaaks and Olsson70–Reference Tamakoshi, Toyoshima and Wakai72), and adiponectin, which has antiangiogenic and antitumour activities(Reference Brakenhielm, Veitonmaki, Cao, Kihara, Matsuzawa, Zhivotovsky, Funahashi and Cao73–Reference Lukanova, Soderberg, Kaaks, Jellum and Stattin75). However, the exact role of these adipokines in the risk of colon cancer remains to be defined.
Obesity and breast cancer
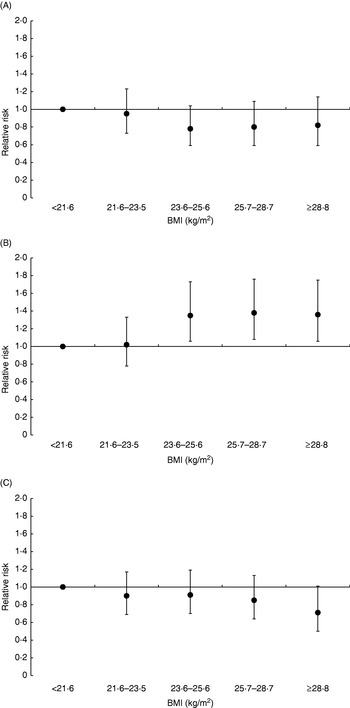
According to recent estimates 429 900 cases of breast cancer were diagnosed in Europe in 2006, making breast cancer not only the most frequent type of cancer in women (28·9% of all female incident cancers) but also the most frequent type of cancer in the whole European population(Reference Ferlay, Autier, Boniol, Heanue, Colombet and Boyle17). Similarly, with 131 900 deaths in 2006, breast cancer is the most frequent cause of cancer death among women in Europe (17·6% of all female cancer deaths)(Reference Ferlay, Autier, Boniol, Heanue, Colombet and Boyle17). The association between indicators of body size and risk of breast cancer has been examined in numerous studies(4). Taken together, these studies have provided complex results. In general, BMI and body weight have been found to be positively related to risk of breast cancer among post-menopausal women, whereas inverse associations have been found among premenopausal women. Further, among post-menopausal women the association between BMI and risk of breast cancer has been found to be stronger among women who do not use hormone-replacement therapy (HRT) compared with women who do use hormones. For example, in the EPIC study the relative risk (RR) of breast cancer among post-menopausal women in the highest quintile of BMI compared with the lowest quintile was found to be 1·36 (95% CI 1·06, 1·75) among non-HRT users, whereas no significant association was found among HRT users (Fig. 2)(Reference Lahmann, Hoffmann and Allen76). Among premenopausal women, those in the highest quintile of BMI compared with the lower quintile had a 18% lower risk of breast cancer, although this difference was not significant(Reference Lahmann, Hoffmann and Allen76). Earlier studies, the Nurses’ Health Study(Reference Huang, Hankinson, Colditz, Stampfer, Hunter, Manson, Rosner, Speizer and Willett77), the Women's Health Initiative(Reference Morimoto, White and Chen78) and the Pooling Project(Reference van den Brandt, Spiegelman and Yaun79), have shown similar results. Adult weight gain has generally been associated with greater risk of post-menopausal breast cancer than BMI at a younger age. For example, in the EPIC study post-menopausal women who did not use HRT and had gained >20 kg during adulthood (between age 20 years and approximately age 60 years) were found to have a 52% increased risk of developing post-menopausal breast cancer compared with those with stable weight during adulthood(Reference Lahmann, Schulz and Hoffmann80). As with general obesity, abdominal adiposity (as measured by waist circumference) has also been found to be positively associated with risk of post-menopausal breast cancer, with stronger relationships among non-HRT users than among HRT users(4, Reference Harvie, Hooper and Howell81). However, with the exception of the Nurses' Health Study(Reference Huang, Willett, Colditz, Hunter, Manson, Rosner, Speizer and Hankinson82) most studies have found waist circumference not to be significantly related to post-menopausal breast cancer after adjustment for BMI, indicating that fat distribution is not related to post-menopausal breast cancer beyond adiposity per se(Reference Harvie, Hooper and Howell81). Among premenopausal women, waist circumference has generally not been found to be related to risk of breast cancer(4, Reference Harvie, Hooper and Howell81). Interestingly, however, some studies have found positive associations between waist circumference and premenopausal breast cancer after adjustment for BMI(Reference Lahmann, Hoffmann and Allen76, Reference Harvie, Hooper and Howell81, Reference Huang, Willett, Colditz, Hunter, Manson, Rosner, Speizer and Hankinson82). It is currently unclear whether this outcome reflects a true biological finding or whether it is simply a statistical artifact resulting from the high collinearity between waist circumference and BMI.

Fig. 2. Relative risk of breast cancer according to quintiles of BMI in premenopausal women (A), in post-menopausal women who did not use hormone-replacement therapy (HRT; B), and in post-menopausal women who reported use of HRT (C) during a mean follow-up of 4·7 years of 176 886 women from the European Prospective Investigation into Cancer and Nutrition(Reference Lahmann, Hoffmann and Allen76). Relative risks for BMI were adjusted for age, study centre, smoking status, education, alcohol intake, parity, age at first pregnancy and age at menarche. Relative risks for premenopausal women were additionally adjusted for use of oral contraceptives. Values are means and 95% CI represented by vertical bars. For premenopausal women P=0·19 for trend, for post-menopausal non-HRT users P=0·002 for trend and for post-menopausal HRT users P=0·07 for trend.
The mechanisms that underlie the association between obesity and breast cancer risk are not completely understood but several hypotheses have been proposed, including alterations in sex hormones, growth factors and cytokines. The adipose tissue expresses sex steroid-metabolizing enzymes that promote the formation of oestrogens from androgenic precursors. After menopause, when ovarian oestrogen production is suspended, the adipose tissue becomes the major source of endogenous oestradiol(Reference Siiteri83, Reference Azziz84). Obese post-menopausal women have higher conversion rates of sex hormones compared with non-obese post-menopausal women. Further, obesity-related hyperinsulinaemia inhibits hepatic secretion of sex hormone-binding globulin. Both effects result in an increase in bioavailable oestradiol and testosterone in obese post-menopausal women, which through binding to oestrogen and androgen receptors may increase cell proliferation and inhibit apoptosis(Reference Calle and Kaaks66). Plasma levels of free oestradiol and testosterone are positively related to breast cancer incidence in post-menopausal women(Reference Kaaks, Rinaldi and Key85), and it has been shown that the association between obesity and breast cancer risk in post-menopausal women can largely be explained by increased levels of oestrogens, particularly bioavailable oestradiol(Reference Key, Appleby and Reeves86, Reference Rinaldi, Key and Peeters87). Among premenopausal women obesity is associated with a higher frequency of anovulatoric cycles and with lower levels of circulating sex steroid hormones, which may be among the reasons for the observation of an inverse relationship between BMI and premenopausal breast cancer in some studies(Reference Potischman, Swanson, Siiteri and Hoover88). Obesity is also related to reduced levels of IGFBP-1 and -2(Reference Calle and Kaaks66) and consequently increased bioavailability of IGF-1. Insulin and IGF-1 may again both increase cell proliferation and inhibit apoptosis(Reference Koenuma, Yamori and Tsuruo59–Reference Wu, Yakar, Zhao, Hennighausen and LeRoith64). However, the epidemiological evidence of a relationship between plasma levels of IGF-1 and its binding proteins and risk of breast cancer has been inconsistent(Reference Renehan, Zwahlen, Minder, O'Dwyer, Shalet and Egger67, Reference Schernhammer, Holly, Pollak and Hankinson89–Reference Shi, Yu, McLarty and Glass94). More recent studies suggest that adipose tissue-derived hormones, including adiponectin and leptin, may also be directly involved in breast cancer development(Reference Tworoger, Eliassen, Kelesidis, Colditz, Willett, Mantzoros and Hankinson95–Reference Miyoshi, Funahashi, Kihara, Taguchi, Tamaki, Matsuzawa and Noguchi100).
Obesity and endometrial cancer
With an estimated 149 300 newly-diagnosed cases of uterine cancer in Europe in 2006, this type of cancer accounted for 10·0% of cancer incidence in women(Reference Ferlay, Autier, Boniol, Heanue, Colombet and Boyle17). Within the same year 46 600 women died of uterine cancer, thereby accounting for 6·2% of cancer deaths in women(Reference Ferlay, Autier, Boniol, Heanue, Colombet and Boyle17). Adult obesity is associated with a 2- to 3-fold increased risk of endometrial cancer, and about 40% of endometrial cancer incidence has been estimated to be attributable to excess body weight(Reference Bergstrom, Pisani, Tenet, Wolk and Adami101). In 2002–3 the International Agency for Research on Cancer expert panel judged the evidence for this association in twenty-five case–control and cohort studies as being sufficient(4). However, in this evaluation it remained unclear whether the association between body weight and risk of endometrial cancer was linear, or whether it was restricted to overweight or obese women(4). Some of the inconsistencies across studies may have been attributable to the use of weight or BMI to classify obesity, which has been shown to be an imperfect measure of adiposity (see earlier discussion). The inconsistencies could also be a result of variations in body fat distribution between the different study populations or of potential differences in the underlying biological mechanisms between premenopausal women v. post-menopausal women. In obese women before the menopause it is probably primarily the lack of progesterone (because of ovarian androgen production and continuous anovulation) that may increase the risk of endometrial cancer, whereas after the menopause excess weight may continue to increase risk primarily through elevated plasma levels of bioavailable oestrogens in the absence of ovarian progesterone synthesis (see later)(Reference Kaaks, Lukanova and Kurzer65). Adult weight gain, potentially a more important indicator of long-term energy balance, has been shown to be associated with increases in risk for endometrial cancer in a dose-dependent manner(Reference Le Marchand, Wilkens and Mi102–Reference Terry, Baron, Weiderpass, Yuen, Lichtenstein and Nyren106). Some evidence that fat distribution may be important for endometrial cancer has emerged from studies that have looked at other measures of adiposity, including waist:hip ratio, waist:thigh ratio, subscapular skinfold and subscapular:thigh skinfold ratio. An association for waist:hip ratio independent of BMI has been shown in five case–control studies(Reference Swanson, Potischman, Wilbanks, Twiggs, Mortel, Berman, Barrett, Baumgartner and Brinton104,Reference Elliott, Matanoski, Rosenshein, Grumbine and Diamond107–Reference Iemura, Douchi, Yamamoto, Yoshimitsu and Nagata110), whereas another five studies, including two cohort studies(Reference Folsom, Kaye, Potter and Prineas111, Reference Lapidus, Helgesson, Merck and Bjorntorp112), have not shown such an independent association(Reference Shu, Brinton, Zheng, Swanson, Hatch, Gao and Fraumeni103,Reference Folsom, Kaye, Potter and Prineas111–Reference Goodman, Hankin, Wilkens, Lyu, McDuffie, Liu and Kolonel114). Subscapular skinfold measures have been shown to better predict endometrial cancer risk than waist:hip ratio and independent of BMI in two case–control studies(Reference Shu, Brinton, Zheng, Swanson, Hatch, Gao and Fraumeni103, Reference Austin, Austin, Partridge, Hatch and Shingleton113).
The associations between measures of obesity and risk of endometrial cancer have also been investigated in the EPIC Study that included 223 008 women(Reference Friedenreich, Cust and Lahmann115). In that analysis 567 cases of endometrial cancer were identified over a mean follow-up of 6·4 years(Reference Friedenreich, Cust and Lahmann115). Compared with normal-weight women, obese and morbidly-obese women (BMI ≥40 kg/m2) were found to have a significantly increased RR of 1·78 (95% CI 1·41, 2·26) and 3·02 (95% CI 1·66, 5·52) of developing endometrial cancer respectively. In contrast, overweight women were not found to be at increased risk (RR 1·11 (95% CI 0·91, 1·36)), although the trend across BMI categories was found to be highly significant (P<0·0001 for trend). These findings support the possibility of a threshold effect of BMI on endometrial cancer risk. Waist circumference, hip circumference and waist:hip ratio were all found to be positively and significantly associated with endometrial cancer risk. However, after additional adjustment for BMI, only the RR for waist circumference ≥88 cm compared with <80 cm remained significant (1·50 (95% CI 1·10, 2·04); P=0·02 for trend), indicating that abdominal body fat may aetiologically be more relevant than gluteofemoral body fat(Reference Friedenreich, Cust and Lahmann115). Data on weight change during adulthood were available for a subcohort of 264 cases and 106 272 non-cases. An elevated RR of 1·75 (95% CI 1·11, 2·77) was calculated for women who had gained ≥20 kg between age 20 years and the time of enrolment into the study (approximately age 50 years) compared with women who had stable weight (±3 kg) during this time period(Reference Friedenreich, Cust and Lahmann115). A risk increase of 13% was estimated for a gain in weight of 5 kg(Reference Friedenreich, Cust and Lahmann115). The associations between weight, BMI and hip circumference and endometrial cancer were found to be stronger for post-menopausal women than for premenopausal women, while for the association between waist circumference and waist:hip ratio and endometrial cancer somewhat greater risks were found for premenopausal women than for post-menopausal women. However, these differences were not found to be significant (P≥0·10 for all interactions). In contrast, evidence for an interaction between adiposity and HRT use on endometrial cancer risk was observed, such that among ‘never-users’ of HRT weight, BMI and waist and hip circumferences were significantly associated with risk of endometrial cancer, whereas no significant associations were observed among ‘ever-users’ of HRT. No interaction was observed between measures of obesity and use of oral contraceptives on risk of endometrial cancer, although the associations were slightly stronger in ‘never-users’ than ‘ever-users’.
Alterations in endogenous hormone metabolism may provide the main links between obesity and endometrial cancer risk(Reference Kaaks, Lukanova and Kurzer65). The ‘unopposed estrogen’ hypothesis proposes that endometrial cancer may develop as a result of the mitogenic effects of oestrogens when these are insufficiently counterbalanced by progesterone. Hence, endometrial cancer risk is supposed to be increased in women who have high plasma levels of bioavailable oestrogens and/or low progesterone levels. It was proposed that elevated oestrogens and low progesterone promotes the development and growth of endometrial tumours largely through the increase in IGF-1 bioactivity within endometrial tissue, resulting from oestrogen-induced IGF-1 synthesis and reductions in IGFBP-1 because of lack of progesterone(Reference Kaaks, Lukanova and Kurzer65). Further risk factors for endometrial cancer related to endogenous hormone metabolism are low plasma sex hormone-binding globulin, elevated plasma androgens and elevated insulin levels(Reference Kaaks, Lukanova and Kurzer65). Excess weight has been linked to most of these hormonal changes(Reference Kaaks, Lukanova and Kurzer65). Obesity is generally associated with insulin resistance, leading to elevated plasma insulin levels, which affect the IGF-I–IGFBP system (see earlier discussion). For example, prediagnostic levels of C-peptide have been shown to be associated with increased endometrial cancer risk (RR for the highest quintile compared with the lowest quintile 4·76 (95% CI 1·91, 11·8))(Reference Lukanova, Zeleniuch-Jacquotte and Lundin116). Furthermore, excess weight leads to a decrease in plasma sex hormone-binding globulin, a rise in oestrogens and a rise in specific androgens(Reference Kaaks, Lukanova and Kurzer65). In a multicentre prospective study in post-menopausal women circulating oestrogens and androgens were found to be positively associated with endometrial cancer risk, and an inverse association was reported for sex hormone-binding globulin(Reference Lukanova, Lundin and Micheli117).
Obesity and renal cell cancer
The incidence of kidney cancer is increasing worldwide(Reference Mathew, Devesa, Fraumeni and Chow118). In the EU it was estimated that in 2006 kidney cancer accounted for 3·1% of total cancer incidence in men and for 2·3% of total cancer incidence in women, while it was the cause of 2·5% of deaths from cancer in men and of 2·0% of deaths from cancer in women(Reference Ferlay, Autier, Boniol, Heanue, Colombet and Boyle17). In absolute numbers, 39 400 men and 24 000 women were newly diagnosed with kidney cancer in the EU in 2006, and 16 200 men and 10 200 women died because of this disease(Reference Ferlay, Autier, Boniol, Heanue, Colombet and Boyle17). Renal cell carcinoma is the major type (80–90%) of kidney cancer, whereas renal pelvis cancer is a rare type of cancer, originating from the transitional cell epithelium within the kidney, that resembles ureter and bladder cancer(Reference Vogelzang and Stadler119). US studies suggest that the increase in kidney cancer incidence can only partly be explained by improved detection of asymptomatic tumours(Reference Chow, Devesa, Warren and Fraumeni120). Thus, more detailed analyses have revealed that incidence rates for renal cell carcinoma have increased largely independent of tumour stage, whereas incidence rates for renal pelvis cancer have not increased(Reference Chow, Devesa, Warren and Fraumeni120). The reasons for the observed increased incidence rates of renal cell carcinoma are unclear but may include the rising prevalence of obesity. The relationship between BMI, body weight and risk of renal cell carcinoma has been examined in several studies(Reference McLaughlin, Mandel, Blot, Schuman, Mehl and Fraumeni121–Reference Bjorge, Tretli and Engeland152). Most of these studies have established obesity as a risk factor for renal cell cancer, and the WHO report has concluded that there is sufficient evidence that obesity increases the risk of this type of cancer(4, 5). However, there are some uncertainties. For example, earlier reviews have suggested that the association between body weight, BMI and risk of renal cell carcinoma may be stronger in women than in men(Reference Wolk, Lindblad and Adami153, Reference McLaughlin and Lipworth154), although a subsequent meta-analysis has found that the relationship is equally strong in both genders(Reference Bergstrom, Hsieh, Lindblad, Lu, Cook and Wolk155). In contrast, within EPIC it was recently observed that a high BMI is a risk factor for renal cell cancer in women but not in men(Reference Pischon, Lahmann and Boeing156). Thus, during an average 6·0-year follow-up of 348 550 participants the RR of developing renal cell cancer in individuals with a BMI of ≥30 kg/m2 compared with those with a BMI of <25 kg/m2 was found to be 1·68 (95% CI 1·03, 2·75) among women and 1·06 (95% CI 0·66, 1·70) among men. The reasons for these gender differences are unclear. The association between body fat distribution and risk of renal cell cancer has been examined in only a few studies, and results from these studies suggest that fat distribution does not predict renal cell cancer risk beyond adiposity in general(4, Reference Pischon, Lahmann and Boeing156).
The mechanisms that link overweight and obesity with renal cell carcinoma are only poorly understood. One popular hypothesis is that obesity may increase risk of renal cell carcinoma by affecting plasma levels of bioavailable IGF-1(Reference Calle and Kaaks66, Reference Chow, Gridley, Fraumeni and Jarvholm150). Nevertheless, although IGF-1 is known to have cancer-promoting activities and has been shown to be related to other types of cancer(Reference Giovannucci53, Reference Calle and Kaaks66), the hypothesis that IGF-1 is related to risk of renal cell carcinoma has not been tested in human studies. Obesity is also related to an increased risk of hypertension and diabetes, both of which are risk factors for renal cell cancer(Reference Vogelzang and Stadler119, Reference Chow, Gridley, Fraumeni and Jarvholm150, Reference Lindblad, Chow, Chan, Bergstrom, Wolk, Gridley, McLaughlin, Nyrén and Adami157). However, limited lines of evidence suggest that obesity increases risk of renal cell cancer even independently of blood pressure levels(Reference Chow, Gridley, Fraumeni and Jarvholm150). Experimental and observational data suggest that obesity-related biomarkers may also be involved in tumourigenesis and tumour progression(Reference Brakenhielm, Veitonmaki, Cao, Kihara, Matsuzawa, Zhivotovsky, Funahashi and Cao73,Reference Wei, Giovannucci, Fuchs, Willett and Mantzoros74, Reference Mantzoros, Petridou and Dessypris97, Reference Rose, Komninou and Stephenson99, Reference Miyoshi, Funahashi, Kihara, Taguchi, Tamaki, Matsuzawa and Noguchi100, Reference Tessitore, Vizio, Jenkins, De Stefano, Ritossa, Argiles, Benedetto and Mussa158–Reference Petridou, Mantzoros, Dessypris, Koukoulomatis, Addy, Voulgaris, Chrousos and Trichopoulos161); however, little is known about the relationship between these biomarkers and risk of renal cell carcinoma. Very recently, lower adiponectin levels were observed in individuals with renal cell cancer when compared with healthy controls(Reference Spyridopoulos, Petridou, Skalkidou, Dessypris, Chrousos and Mantzoros162). Interestingly, this association remained significant when differences in BMI between individuals were taken into account but became non-significant when accounting for waist:hip ratio. Clearly, prospective studies are needed to examine the role of obesity biomarkers in the development of renal cell cancer.
Obesity and oesophageal cancer
It was estimated that 34 300 men and 10 700 women were newly diagnosed with oesophageal cancer in Europe in 2006 (2·0% of total cancer incidence in men and 0·7% of total cancer incidence in women)(Reference Ferlay, Autier, Boniol, Heanue, Colombet and Boyle17). Within the same year, approximately 29 300 men and 9200 women died from oesophageal cancer (3·1% of cancer deaths in men and 1·2% of cancer deaths in women)(Reference Ferlay, Autier, Boniol, Heanue, Colombet and Boyle17). Strikingly, the occurrence for this type of cancer is 3-fold higher in men than in women. The major histological types of oesophageal cancer are squamous cell carcinoma and adenocarcinoma. Squamous cell carcinoma is the predominant type of oesophageal cancer and has been clearly linked to smoking tobacco and drinking alcohol(Reference Munoz, Day, Schottenfeld and Fraumeni163). In industrialized countries the incidence of oesophageal squamous cell carcinoma has remained relatively constant or even declined during previous decades(4, Reference Brown and Devesa164). Oesophageal squamous cell carcinomas predominantly occur in the upper and middle part of the oesophagus, while oesophageal adenocarcinomas most frequently occur in the lower part of the oesophagus. However, it is often difficult to differentiate whether an adenocarcinoma originated in the distal oesophagus or in the gastro-oesophageal junction and gastric cardia(Reference Chandrasoma, Wickramasinghe, Ma and DeMeester165). The problem of proper organ assignment of the clinically-apparent cancer may have influenced the epidemiological findings and may also have contributed to the idea that oesophageal adenocarcinomas share time trends and common risk factors with adenocarcinomas of the gastric cardia(Reference Souza and Spechler166). Further, in contrast to squamous cell carcinomas, the incidence of oesophageal adenocarcinomas has been increasing in Western societies during previous decades(Reference Pera, Manterola, Vidal and Grande167). This rise in incidence has partly been attributed to the rise in the prevalence of obesity. In fact, one of the surprising results from the expert evaluation in 2002–3 was a consistent finding of an increased risk of oesophageal adenocarcinoma with increasing BMI, although this finding was largely based on case–control studies(4). The link between obesity and risk of oesophageal adenocarcinoma has recently been confirmed by a quantitative meta-analysis that included twelve case–control studies and two cohort studies(Reference Kubo and Corley168). For overweight and obese subjects OR for oesophageal adenocarcinomas of 1·8 (95% CI 1·5, 2·2) and 2·4 (95% CI 1·9, 3·2) respectively among men and 1·5 (95% CI 1·1, 2·2) and 2·1 (95% CI 1·4, 3·2) respectively among women compared with normal-weight individuals were found in this analysis(Reference Kubo and Corley168). Interestingly, analyses of prospective data from Norway that confirmed the link between obesity and oesphageal adenocarcinoma have found inverse associations between BMI and oesophageal squamous cell carcinoma(Reference Tretli and Robsahm169, Reference Engeland, Tretli and Bjorge170). Given the strong effect of smoking on oesophageal squamous cell carcinoma and given the known association between smoking and lower BMI it is currently unclear whether the observed inverse association between BMI and oesophageal squamous cell carcinoma reflects a causal protective relationship or whether this association is rather a result of residual confounding because of incomplete adjustment for smoking status. In contrast, more is known about the potential factors causing the increase in oesophageal adenocarcinoma risk with increasing BMI. In a recent systematic review obesity was found to be related to gastroesophageal reflux as well as to oesophagitis in most of the included studies(Reference Hampel, Abraham and El-Serag171). Gastroesophageal reflux itself is considered to be a major risk factor for oesophageal adenocarcinoma, both alone and in combination with obesity(Reference Mayne, Risch and Dubrow172). Further, obesity is related to oesophagitis, and this association can only partly be explained by clinical apparent gastroesophageal reflux in obese individuals. For example, in a recent prospective study oesophagitis was found to be related to a 5-fold increased risk of adenocarcinoma of the oesophagus(Reference Lassen, Hallas and de Muckadell173). Most adenocarcinomas of the oesophagus develop on the basis of Barrett's oesophagus, which is considered to be a premalignant condition characterized by replacement of squamous epithelium with columnar epithelium(Reference Flejou174). The exact role that obesity plays within these processes remains to be defined.
Obesity and pancreatic cancer
Pancreatic cancer was estimated to account for 2·5% of incident cancers and 5·5% of cancer deaths in Europe in 2006(Reference Ferlay, Autier, Boniol, Heanue, Colombet and Boyle17). The estimated 5-year survival rate of patients with pancreatic cancer is 5%(175). Its high case fatality rate renders pancreatic cancer a difficult cancer to study since case–control studies (prone to recall bias in the first place) largely have to rely on proxy interviews. Further, pancreatic cancer is often accompanied by severe tumour cachexia, leading to substantial weight loss even before diagnosis(Reference Li, Xie, Wolff and Abbruzzese176), which may bias any association between body weight and cancer risk when examined in case–control studies. On the other hand, prospective studies need to follow-up large numbers of individuals for longer time periods to accrue a sufficient number of incident pancreatic cancer cases in order to achieve appropriate power for meaningful analyses. In fact, early evidence for an association between obesity and pancreatic cancer risk from case–control studies is weak, and is now, in light of new evidence from prospective cohort studies, believed to have been biased because of high case fatality or reliance on proxy interviews(Reference Giovannucci and Michaud177). A considerable number of prospective studies have examined associations between measures of obesity and pancreatic cancer during the last years(Reference Samanic, Gridley, Chow, Lubin, Hoover and Fraumeni178–Reference Luo, Iwasaki, Inoue, Sasazuki, Otani, Ye and Tsugane200), and a recent meta-analysis (based on results of twenty-one independent prospective studies involving a total of 3 495 981 individuals and 8062 incident pancreatic cancer cases over a mean follow-up of 13·5 years) has estimated a summary RR of pancreatic cancer per 5 kg/m2 increase in BMI of 1·16 (95% CI 1·05, 1·28) for men and of 1·10 (1·02–1·19) for women(Reference Larsson, Orsini and Wolk201).
A recent analysis from the EPIC study has reported a positive non-significant association between BMI and pancreatic cancer risk (RR 1·09 (95% CI 0·95, 1·24) per 5 kg/m2)(Reference Berrington de Gonzalez, Spencer and Bueno-de-Mesquita182). This study analysed data from 324 incident pancreatic cancer cases observed among 438 405 participants during a follow-up of 6 years(Reference Berrington de Gonzalez, Spencer and Bueno-de-Mesquita182). The evaluation of measured waist:hip ratio or waist circumference, however, revealed significantly positive associations; RR were 1·13 (95% CI 1·01, 1·26) for a 10 cm increase in waist circumference and 1·24 (95% CI 1·04, 1·48) for a 0·1 increase in waist:hip ratio. The RR estimates differed somewhat between males and females, but none of the interactions with gender were significant at the P<0·05 level. Interestingly, when individuals were excluded who were diagnosed with pancreatic cancer during the first 2 years to reduce the possible effect of prediagnostic symptoms (including tumour cachexia) the associations between waist circumference and waist:hip ratio and risk of pancreatic cancer became even stronger. The positive significant association between waist circumference and pancreatic cancer has recently been confirmed in a combined analysis of cohort studies for the Asia-Pacific region (RR 1·08 (95% CI 1·02, 1·14) for a 2 cm increase in waist circumference)(Reference Ansary-Moghaddam, Huxley, Barzi, Lawes, Ohkubo, Fang, Jee and Woodward202). As in the EPIC Study(Reference Berrington de Gonzalez, Spencer and Bueno-de-Mesquita182), BMI was not found to be significantly associated with pancreatic cancer in this analysis(Reference Ansary-Moghaddam, Huxley, Barzi, Lawes, Ohkubo, Fang, Jee and Woodward202). Two further studies have found some evidence for a positive association with waist circumference in men, but not in women(Reference Sinner, Schmitz, Anderson and Folsom185, Reference Larsson, Permert, Hakansson, Naslund, Bergkvist and Wolk190). Self-reported central weight gain compared with peripheral weight gain has also been associated with increased pancreatic cancer risk(Reference Patel, Rodriguez, Bernstein, Chao, Thun and Calle183).
Accumulating evidence supports a role of factors related to hyperinsulinaemia and hyperglycaemia in the pathophysiology of pancreatic cancer. A recent meta-analysis of thirty-six studies has found that subjects with diabetes have a 1·82-fold increased risk for pancreatic cancer compared with individuals who do not have diabetes (95% CI 1·66, 1·89), thus supporting the hypothesis that diabetes mellitus is associated with elevated pancreatic cancer risk(Reference Huxley, Ansary-Moghaddam, Berrington de Gonzalez, Barzi and Woodward203). The notion that factors associated with abnormal glucose metabolism may promote the development of pancreatic carcinoma has further been supported by epidemiological studies showing positive associations between elevations in fasting serum glucose or post-load plasma glucose and risk of pancreatic cancer(Reference Gapstur, Gann, Lowe, Liu, Colangelo and Dyer189, Reference Jee, Ohrr, Sull, Yun, Ji and Samet204). To date, only one study has investigated the association between pre-diagnostic serum insulin levels and risk of pancreatic cancer; it has shown an RR of 2·01 (95% CI 1·03, 3·93) for the highest quartile of insulin levels v. the lowest quartile(Reference Stolzenberg-Solomon, Graubard, Chari, Limburg, Taylor, Virtamo and Albanes205). A role of IGF in the pathophysiology of pancreatic cancer has also been hypothesized. However, a nested case–control study within four large cohort studies has reported no significant associations between pre-diagnostic plasma levels of IGF-1, IGF-2 and IGFBP-3 and pancreatic cancer risk(Reference Wolpin, Michaud and Giovannucci206). Similarly, two smaller studies that have evaluated the associations between IGF-1 and IGFBP-3 and risk of pancreatic cancer have found no significant associations(Reference Stolzenberg-Solomon, Limburg, Pollak, Taylor, Virtamo and Albanes207, Reference Lin, Tamakoshi and Kikuchi208). Since the insulin pathway also interacts with the IGF axis the latter might still play a role in the pathogenesis of pancreatic cancer, although the evidence from prospective epidemiological studies has not been assuring so far(Reference Giovannucci209).
Obesity and prostate cancer
Prostate cancer is the most common cancer diagnosed in men in Europe(Reference Ferlay, Autier, Boniol, Heanue, Colombet and Boyle17). For 2006 it was estimated that 345 900 men were diagnosed with prostate cancer (20·3% of all male incident cancers)(Reference Ferlay, Autier, Boniol, Heanue, Colombet and Boyle17). With 87 400 deaths in 2006, prostate cancer accounted for 9·2% of all male cancer deaths(Reference Ferlay, Autier, Boniol, Heanue, Colombet and Boyle17). The incidence of prostate cancer strongly depends on age, as it is only rarely diagnosed among men younger than 50 years (<0·1% of all patients), whereas the majority of patients (85%) are older than 65 years(Reference Gronberg210). The cumulative risk of developing prostate cancer by age 85 years is estimated to be up to 20%(Reference Gronberg210). As a result of improvements in diagnostic procedures and increased screening in most countries, prostate cancer is currently usually diagnosed at the earlier stages (i.e. more organ-confined disease) than previously(Reference Moul, Wu and Sun211–Reference Jang, Han, Roehl, Hawkins and Catalona213). Epidemiological studies that have examined the association between obesity and risk of prostate cancer have provided conflicting results. With a few exceptions, most studies have failed to show overall significant associations between BMI and risk of prostate cancer(4), although a recent meta-analysis has suggested a weak significant positive association, with an estimated increase in prostate cancer risk of 1·05 (95% CI 1·01, 1·08) per 5 kg/m2(Reference MacInnis and English214). However, some studies have suggested that when separated by stage of disease or by tumour grade obesity may be strongly related to a higher risk of advanced-stage prostate cancer and of high-grade tumours but not, or even inversely, related to early-stage (i.e. localized) prostate cancer and to low-grade tumours. This finding is also supported by the meta-analysis, which has found stronger associations for advanced-stage prostate cancer (estimated RR of 1·12 (95% CI 1·01, 1·23) per increase in BMI of 5 kg/m2) compared with localized disease (RR 0·96 (95% CI 0·89, 1·03) per 5 kg/m2)(Reference MacInnis and English214). Results from more recent cohort studies have provided further support to this hypothesis(Reference Gong, Neuhouser and Goodman215, Reference Rodriguez, Freedland, Deka, Jacobs, McCullough, Patel, Thun and Calle216). For example, in the Prostate Cancer Prevention Trial men in the highest quartile of BMI were found to have a 1·29-fold increased risk (95% CI 1·01, 1·67; P=0·04 for trend) for high-grade cancer but a 0·91-fold decreased risk (95% CI 0·69, 0·98; P=0·03 for trend) for low-grade cancer(Reference Gong, Neuhouser and Goodman215). Similarly, in the Cancer Prevention Study II risk of non-metastatic low-grade prostate cancer was found to be decreased significantly with increasing BMI (P=0·002 for trend), whereas the risk of non-metastatic high-grade prostate cancer was increased significantly with increasing BMI (P=0·03 for trend)(Reference Rodriguez, Freedland, Deka, Jacobs, McCullough, Patel, Thun and Calle216). The strongest associations have been found in studies that have examined the association between BMI and metastatic or fatal prostate cancer. For example, in the previously mentioned Cancer Prevention Study II men with a BMI of ≥30 kg/m2 were found to have a 1·54-fold increase in risk (95% CI 1·06, 2·23) of developing metastatic or fatal prostate cancer compared with men with a BMI of <25 kg/m2(Reference Rodriguez, Freedland, Deka, Jacobs, McCullough, Patel, Thun and Calle216). Age may be an additional factor that modifies the association between obesity and prostate cancer. Thus, in the Health Professionals Follow-up Study BMI was found to be significantly inversely related to prostate cancer in men aged <60 years, whereas no such association was observed in men aged >60 years (P<0·0001 for interaction)(Reference Giovannucci, Rimm, Liu, Leitzmann, Wu, Stampfer and Willett217). The association between waist circumference or waist:hip ratio and risk of prostate cancer has been examined in only a very few studies(Reference MacInnis and English214, Reference Giovannucci, Rimm, Liu, Leitzmann, Wu, Stampfer and Willett217–Reference Hsing, Deng, Sesterhenn, Mostofi, Stanczyk, Benichou, Xie and Gao221), with most studies finding no significant association. Clearly, further studies are needed to examine in more detail the association between body fat distribution and risk of prostate cancer.
It is unclear why obesity is related to lower risk of early-stage low-grade prostate cancer but to a higher risk of late-stage high-grade disease; however, several hypotheses have been put forward, including both biological and non-biological mechanisms(Reference Freedland, Giovannucci and Platz222, Reference Freedland and Platz223). Thus, it is known that obese men have increased serum oestradiol levels but decreased testosterone levels compared with non-obese men. Androgens are required for the growth, maturation and differentiation of the prostate gland(Reference Marker, Donjacour, Dahiya and Cunha224, Reference Parnes, Thompson and Ford225) It has thus been suggested that testosterone may promote prostate tumour development but may also help maintain prostate tumour differentiation(Reference So, Hurtado-Coll and Gleave226), which may explain why obese individuals with low testosterone levels have a higher risk of developing undifferentiated tumours(Reference Freedland, Giovannucci and Platz222, Reference Freedland and Platz223). Other mechanisms that may link obesity with prostate cancer may include high insulin levels, high bioavailable IGF-1 levels, high leptin levels or low adiponectin levels, although most prospective studies on this topic have provided inconsistent results(Reference Renehan, Zwahlen, Minder, O'Dwyer, Shalet and Egger67, Reference Shaneyfelt, Husein, Bubley and Mantzoros227–Reference Baillargeon, Platz and Rose236). Also, these latter hormonal mechanisms may not easily explain the difference in the association between obesity and low-grade early-stage disease compared with its association with high-grade late-stage disease. Alternatively, or additionally, the fact that obese individuals have a higher risk of high-grade late-stage prostate cancer but a lower risk of low-grade early-stage disease may also be explained by delayed detection and diagnosis of prostate cancer in obese individuals(Reference Freedland, Giovannucci and Platz222, Reference Freedland and Platz223). Proposed reasons for difficulties in prostate cancer detection in obese individuals include lower prostate-specific antigen levels and larger prostate sizes in obese men when compared with non-obese men, as well as the fact that a digital rectal examination may be more difficult to perform in obese men(Reference Freedland, Giovannucci and Platz222, Reference Freedland and Platz223).
Obesity and other types of cancer
Obesity has also been linked to other types of cancer, although overall the amount of data available is limited and does not allow definite conclusions. Particular interest has recently been devoted to gallbladder and liver cancer. In industrialized countries these two cancers are relatively rare when compared with other types; therefore, gallbladder and liver cancer probably have not received much attention in large-scale prospective studies in the past. The relationship between obesity and risk of gallbladder cancer has recently been investigated in a meta-analysis(Reference Larsson and Wolk237) that included eight cohort studies and three case–control studies with a total of 3288 cases. Compared with individuals of normal weight the RR of gallbladder cancer was found to be increased 1·15-fold in those who were overweight (95% CI 1·01, 1·30), and 1·66-fold in those who were obese (95% CI 1·47, 1·88). For individuals with obesity the RR was stronger for women (1·88 (95% CI 1·66, 2·13)) than for men (1·35 (95% CI 1·09, 1·68)). The mechanisms by which obesity may affect gallbladder cancer risk are unclear as yet. However, gallstone formation is a major risk factor for this disease and obesity is one of the factors that increases gallstone formation(Reference Randi, Franceschi and La Vecchia238). Cholecystectomy is often the treatment of choice for gallstone formation, and it was shown that the risk of cholecystectomy increases with higher BMI and also independently with waist circumference and waist:hip ratio(Reference Tsai, Leitzmann, Willett and Giovannucci239). Obesity as well as type 2 diabetes are also likely to be risk factors for hepatocellular cancer, the most frequent subtype of liver cancer(Reference Caldwell, Crespo, Kang and Al-Osaimi240). The main pathway by which obesity probably increases risk probably relates to the association between obesity and non-alcoholic fatty liver disease(Reference Clark241). Non-alcoholic fatty liver disease is increasingly frequently seen in Western societies. It is linked to insulin resistance, oxidative stress and obesity, and it can progress to steatohepatitis and to cirrhosis. Most cases of hepatocellular cancer seen in the USA and Europe are likely to have a background of non-alcoholic steatohepatitis with cirrhosis(Reference Caldwell, Crespo, Kang and Al-Osaimi240).
Obesity has also been linked to increased mortality from non-Hodgkin's lymphoma, multiple myeloma and leukaemia, as well as from cancers of the cervix and ovaries(Reference Calle, Rodriguez, Walker-Thurmond and Thun180). However, only a few studies have examined the association between overweight and obesity and incidence of these malignancies, and most of these studies have been restricted to case–control designs or have included only small numbers of cases. A recent systematic review, based on twenty-eight eligible studies, has found overweight to be associated with a 1·2-fold increased risk (95% CI 1·0, 1·3) of ovarian cancer and obesity to be related to a 1·3-fold increased risk (95% CI 1·1, 1·5) of ovarian cancer(Reference Olsen, Green, Whiteman, Sadeghi, Kolahdooz and Webb242). Another systematic review, based on sixteen eligible studies, has found a significantly increased RR of non-Hodgkin's lymphomas for overweight (1·07 (95% CI 1·01, 1·14)) and obese (1·20 (95% CI 1·07, 1·34)) individuals(Reference Larsson and Wolk243). A case–control study performed in the USA has found obesity to be related to a 2·1-fold risk (95% CI 1·1, 3·8) of adenocarcinomas of the cervix but not related to squamous cell carcinomas of the cervix (RR 1·6 (95% CI 0·84, 2·9))(Reference Lacey, Swanson and Brinton244). A study from Norway has found obesity to be related to risk of lymphohaematopoietic malignancies, including Hodgkin's lymphoma and non-Hodgkin's lymphoma, acute and chronic lymphatic leukaemia and acute and chronic myeloid leukaemia(Reference Engeland, Tretli, Hansen and Bjorge245). Clearly, more prospective studies are needed to investigate the association between obesity and these malignant tumour sites in more detail. Furthermore, the association between body fat distribution variables, including waist circumference and waist:hip ratio, and risk of these malignancies needs to be investigated.
Conclusions
Among the different cancer sites there is currently sufficient evidence for obesity to increase risk of colon cancer, post-menopausal breast cancer, endometrial cancer, renal cell cancer and adenocarcinoma of the oesophagus(4, 5). The more recently published studies suggest that obesity may also increase risk of other cancer types, including pancreatic cancer, advanced-stage prostate cancer, gallbladder cancer and liver cancer. Among the many dietary factors proposed to be related to cancer incidence, obesity as a sensitive marker of a distorted energy balance is thus among the few factors for which there is sufficient evidence of a relationship with increased cancer risk(5). However, many questions remain. For example, it is currently unclear whether the presumed effect of obesity differs between cancer sites. Studies suggest that the association between obesity and cancer incidence may be stronger for certain types of cancer, including for example endometrial cancer, than for other types of cancer. However, most published studies have focused on one type of cancer, which makes it difficult to compare the strength of the associations between different cancer sites. Furthermore, as noted earlier, men and women differ in their body composition, yet many studies do not present gender-specific results, or they include either men or women; thus rendering it difficult to evaluate whether the associations between obesity and cancer incidence differ between genders. Further complexity is added by the fact that BMI is used in most studies to assess the extent of adiposity. As mentioned earlier, BMI does not take body fat distribution into account and several recent studies have now shown that for certain cancer types, including colon cancer, variables of body fat distribution may be more important, or add further information, for the prediction of cancer. It is also unclear whether the association between body fat and cancer incidence is a linear relationship or whether thresholds exist; for example, some studies have suggested that obesity but not overweight is related to the incidence of certain types of cancer(4). Future studies should therefore systematically examine the shape of the association between the different body size variables (BMI, waist circumference, waist:hip ratio) and risk of the different types of cancer. Furthermore, the underlying mechanisms that link obesity with cancer risk are unclear for most types of cancer. These mechanisms need to be examined in more detail in experimental studies, and supported by observational data that relate relevant biomarkers to cancer risk in human subjects. Such data may also help to further define the obesity phenotype that is relevant for cancer development.
For now, as opposed to other diet-related cancer risk factors, the recommendation to maintain a healthy weight seems to be most promising to support cancer prevention for some cancer sites(Reference Byers, Nestle, McTiernan, Doyle, Currie-Williams, Gansler and Thun246). Elucidation of the shape of the associations, nevertheless, is essential for public health recommendations on how to reduce an individual's cancer risk. Further, measurement of waist circumference or waist:hip ratio should be included in current guidelines to maintain a healthful lifestyle for disease prevention.