Glycerol has been used as a feed ingredient to replace grain in dairy( Reference Chung, Rico and Martinez 1 ) and finishing beef cattle diets( Reference Parsons, Shelor and Drouillard 2 , Reference Mach, Bach and Devant 3 ) and in growing-finishing lamb diets( Reference Avila-Stagno, Chaves and He 4 ). Results suggest that glycerol at 50–100 g/kg DM does not affect weight gain and may improve feed conversion when substituted for barley or maize grain in beef cattle diets( Reference Mach, Bach and Devant 3 ). However, a greater concentration (>120 g/kg diet DM) of glycerol has been reported to result in reduced intakes in cattle( Reference Parsons, Shelor and Drouillard 2 , Reference Mach, Bach and Devant 3 ) and sheep( Reference Avila-Stagno, Chaves and He 4 – Reference Musselman, Van Emon and Gunn 6 ) fed high-grain diets.
Glycerol can increase blood glucose levels in cattle and sheep by being directly absorbed through the rumen wall and converted to glucose in the liver( Reference Rémond, Souday and Jouany 7 ) or by being fermented in the rumen mainly to propionate, which in turn can be absorbed and converted to glucose in the liver( Reference Chung, Rico and Martinez 1 , Reference Johns 8 ). The replacement of wheat starch( Reference Bergner, Kijora and Ceresnakova 9 ) or barley grain( Reference Avila, Chaves and Hernandez 10 ) with glycerol has been reported to linearly increase propionic acid production and reduce the acetate:propionate ratio in vitro. Shifts towards propionate fermentation have been suggested as a means of reducing CH4 emissions, since the metabolic pathways leading to propionate have been proposed as a hydrogen sink( Reference Boadi, Benchaar and Chiquette 11 – Reference McAllister and Newbold 13 ).
Previous studies have reported increases in propionate production with only a numerical decrease of CH4 in vitro ( Reference Avila, Chaves and Hernandez 10 ) (mg CH4/g DM incubated and mg CH4/g DM digested) and in vivo ( Reference Avila-Stagno, Chaves and He 4 ) (g CH4/lamb per d, g CH4/kg DM intake, g CH4/kg DM digested, percentage of gross and digestible energy intake lost as CH4) when glycerol replaced barley grain in finishing lamb diets. A possible cause for this lack of effect is that the shift to propionate fermentation may be of relatively low magnitude given the propiogenic properties of high-starch diets. The findings reported by Rémond et al. ( Reference Rémond, Souday and Jouany 7 ) support this concept, as propiogenesis has been shown to substantially increase when glycerol is added to high-fibre diets than when added to high-starch diets incubated in vitro. CH4 production (ml CH4/g DM incubated) in 24 h in vitro batch cultures has been reported to decrease when glycerol is added to alfalfa- or maize grain-based diets( Reference Lee, Lee and Cho 14 ). Since the acetate:propionate ratio is progressively reduced over a period of 3–4 d after glycerol supplementation in cattle( Reference Rémond, Souday and Jouany 7 , Reference Kijora, Bergner and Gotz 15 ), it is probable that enteric CH4 production may decrease as rumen microbial populations adapt to the inclusion of glycerol in the diet.
As feeding of forage to breeding and growing herds in the beef cattle industry accounts for more than 80 % of greenhouse gas emissions and 55 % of CH4 emissions( Reference Beauchemin, Janzen and Little 16 ), inclusion of glycerol in forage diets is likely to have a greater impact on the reduction of greenhouse gases emitted by beef cattle. We hypothesised that the inclusion of glycerol up to 150 g/kg in forage-based diets may decrease CH4 production in a semi-continuous fermentation system (RUSITEC, rumen simulation technique). Thus, the objective of the present study was to evaluate the effects of adding glycerol to a forage diet in a semi-continuous fermentation apparatus (RUSITEC) on fermentation variables, including CH4 production.
Experimental methods
The present experiment was conducted at the Agriculture and Agri-Food Canada Research Centre in Lethbridge, Alberta, Canada. Donor cows used in the experiment were cared for in accordance with the guidelines of the Canadian Council on Animal Care( Reference Olfert, Cross and McWilliams 17 ).
Experimental design and treatments
The experiment was a complete randomised design with four dietary treatments replicated in two RUSITEC apparatuses( Reference Czerkawski and Breckenridge 18 ). The duration of the experiment was 15 d. The first 8 d were used for adaptation, followed by 7 d of sampling (day 9 to day 15). The experimental treatments included brome hay, maize silage and glycerol in the following proportions: (1) 8·5 g hay+1·5 g maize silage (control); (2) 8·5 g hay+1·0 g maize silage+0·5 g glycerol; (3) 8·5 g hay+0·5 g maize silage+1·0 g glycerol; (4) 8·5 g hay+1·5 g glycerol. These amounts were selected to test inclusions of 50–150 g/kg based on previous in vivo results( Reference Avila-Stagno, Chaves and He 4 ), which indicated DM intake reductions at glycerol concentrations exceeding 140 g/kg.
The ingredients and chemical composition of the substrates are reported in Table 1. Hay and silage were ground through a 4 mm screen (Arthur Thomas Company). Glycerol (99·5 % pure, Sigma–Aldrich) was thoroughly mixed with the hay portion of the diet for each treatment before filling the polyester bags (100 × 200 mm; pore size = 50 μm; B. & S.H. Thompson). Maize silage was incubated in separate bags (50 × 100 mm; pore size = 50 μm).
Table 1 Chemical composition of the substrates
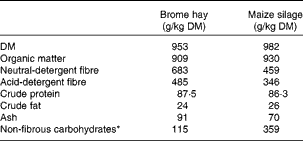
* Calculated as 1000 − (crude protein+neutral-detergent fibre+crude fat+ash).
Experimental apparatuses and incubations
Each RUSITEC apparatus was equipped with eight 920 ml volume anaerobic fermenters. Each fermenter had an inlet for the infusion of buffer and an effluent output port. The fermenters were immersed in a water-bath maintained at 39°C. The four dietary treatments were randomly assigned to duplicate fermenters within each RUSITEC apparatus (four replications per treatment). The experiment was started by filling each fermentation vessel with 180 ml of warmed McDougall's buffer( Reference Fraser, Chaves and Wang 19 ) modified to contain 1·0 g/l of (NH4)2SO4, 720 ml of strained rumen fluid, one bag containing 20 g of wet solid rumen digesta and two additional bags containing the dietary ingredients as described above. After 24 h, the solid rumen digesta bag was replaced with two bags containing each feed. Thereafter, bags that had been incubated for 48 h were replaced daily. Artificial saliva was continuously infused into the fermenters at a dilution rate of 2·9 %/h. During nylon bag exchange, each fermentation vessel was flushed with O2-free CO2 to maintain anaerobic conditions. Effluent accumulation was measured daily during feed bag exchange and collected in a 2·0 litres container containing sodium azide (1 g/l) to arrest microbial growth.
Inoculum was obtained 2 h after feeding from two ruminally cannulated cows fed a forage diet containing barley silage, barley grain and a mineral vitamin supplement (71:25:4 DM basis). Rumen fluid was collected, pooled and filtered through four layers of cheesecloth into an insulated thermos and transported immediately to the laboratory. Approximately 400 g of ruminal solid digesta were also collected for the initial inoculation of the fermenters. Fermentation was initiated in the RUSITEC apparatuses on two consecutive days (two runs).
Sample collection
DM disappearance
DM disappearance at 48 h was determined daily from day 9 to day 15. Feed bags were removed from each fermenter, washed in cold, running distilled water until water was clear, and dried at 55°C for 48 h. To ensure that there was sufficient sample for analysis, silage and concentrate bag residues were pooled over 2 and 3 d, respectively. Samples were ground through a 1 mm screen in a Wiley mill (standard model 4; Arthur H. Thomas) before chemical analysis.
Fermentation metabolites
Fermentation gas was collected into reusable 2-litre vinyl urine collection bags (Bard, Inc.) attached to each fermenter. Just before feed bag exchange, daily total gas production from each fermenter was determined by water displacement( Reference Soliva, Kreuzer and Foidl 20 ). From day 9 to day 15, just before the determination of total gas, gas samples were collected from the septum of the collection bags using a twenty-six-gauge needle (Becton Dickinson). Samples (20 ml) were transferred to evacuated 6·8 ml exetainers (Labco Limited) for immediate analysis of CH4. Fermenter pH was recorded (Orion model 260A, Fisher Scientific) daily at the time of feed bag exchange. To determine the concentration of volatile fatty acids (VFA), subsamples of fermenter liquid (4·0 ml) were collected directly from the fermentation vessels( Reference Fraser, Chaves and Wang 19 ) at the time of feed bag exchange and placed in screw-capped vials preserved with 400 μl of 25 % (w/w) metaphosphoric acid and immediately frozen at − 20°C until analysis. At the same time, 4·0 ml subsamples of fermenter fluid were also collected, placed in screw-capped vials and preserved with 400 μl of TCA until the determination of the concentration of NH3-N. The concentrations of VFA and NH3-N (mmol/l) were multiplied by the outflow rate of fluid infused to the vessels (litres/d) to determine VFA and NH3-N production (mmol/d).
Chemical analysis
Subsamples of each treatment were used for chemical analysis. Feed and fermentation residues were analysed for DM content (method no. 930.15)( 21 ) and ash (method no. 942.05)( 21 ). The concentration of neutral-detergent fibre (NDF) was determined and expressed inclusive of residual ash( Reference Van Soest, Robertson and Lewis 22 ). The concentration of acid-detergent fibre (ADF) was determined according to the method 973.18 (Association of Official Analytical Chemists)( 21 ). The concentration of total N (method no. 990.03)( 21 ) was determined using a mass spectrometer (NA 1500, Carlo Erba Instruments)( Reference Wang, McAllister and Rode 23 ). The concentration of crude fat was determined by diethyl ether extraction (Association of Official Analytical Chemists( 21 ), method 920.39) using the Goldfisch Fat Extractor (Labconco Corporation). The concentrations of VFA and NH3-N in the liquid effluent were determined by GC( Reference Wang, McAllister and Rode 23 ) and the modified Berthelot method( Reference Rhine, Sims and Mulvaney 24 ), respectively. The concentration of CH4 in the gas samples was determined using a Varian gas chromatograph equipped with GS-CarbonPLOT 30 m × 0·32 mm × 3 μm column and thermal conductivity detector (Agilent Technologies Canada, Inc.). Oven temperature was 35°C (isothermal). The carrier gas was helium (27 cm/s), the injector temperature was 185°C (1:30 split, 250 μl injector volume), and the detector temperature was 150°C (thermal conductivity detector).
Statistical analysis
Data were analysed using the MIXED procedure of SAS (SAS, Inc., 2013; SAS Online Doc 9.1.3).
The model included the fixed effects of treatment (substrate), day and treatment × day interactions with the day of sampling from each fermenter treated as a repeated measure. Therefore, the individual fermenter was used as the experimental unit for statistical analysis. The minimum values of Akaike's information criterion were used to select the covariance structure among compound symmetry, heterogeneous compound symmetry, autoregressive, heterogeneous autoregressive, Toeplitz, unstructured and banded for each parameter. Orthogonal polynomial contrasts were carried out to test for linear, quadratic and cubic responses to increasing concentrations of glycerol (0, 50, 100 and 150 g/kg DM) in the substrate. Significance was declared at P≤ 0·05, and a trend was discussed when 0·05 < P< 0·10.
Results
Effects of glycerol on nutrient disappearance
Increasing concentrations of glycerol resulted in a linear increase in DM disappearance from hay (P= 0·001) and maize silage (P= 0·011; Table 2). Crude protein disappearance from hay was not affected (P= 0·788), but that from silage was linearly increased (P< 0·001). Glycerol linearly increased NDF (P= 0·040) and ADF (P= 0·031) disappearance from hay and silage.
Table 2 Effects of increasing concentrations of glycerol on the disappearance of DM, crude protein (CP), neutral-detergent fibre (NDF) and acid-detergent fibre (ADF) of brome hay and maize silage in the rumen simulation technique (Mean values with their standard errors)
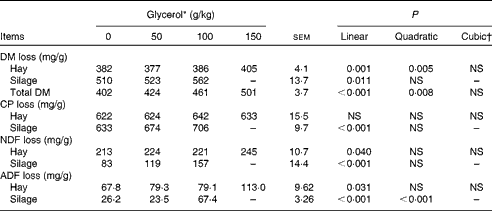
* Experimental substrates: 0 = 8·5 g brome hay+1·5 g maize silage; 50 = 8·5 g brome hay+1·0 g maize silage+0·5 g glycerol; 100 = 8·5 g hay+0·5 g maize silage+1·0 g glycerol; 150 = 8·5 g hay+1·5 g glycerol.
† Cubic contrasts for silage disappearance cannot be calculated since there were only three levels of silage in diet DM.
Effects of glycerol on fermentation
There were no interactions between treatments and sampling day for any of the fermentation variables. The inclusion of glycerol linearly decreased culture pH (P= 0·035) and increased total VFA production (P< 0·001; Table 3). A quadratic effect (P= 0·023) for acetate production was detected with increasing concentrations of glycerol, whereas propionate production was linearly increased (P< 0·001), resulting in a linear and quadratic decline (P< 0·001) in the acetate:propionate ratio. Increasing concentrations of glycerol also resulted in a linear increase in butyrate (P< 0·001) and valerate (P< 0·001) production. The concentration of NH3 was linearly reduced by the addition of glycerol (P< 0·001), although the magnitude of the effect was small.
Table 3 Effects of increasing concentrations of glycerol on the fermentation characteristics of a brome hay–maize silage diet in the rumen simulation technique (Mean values with their standard errors)
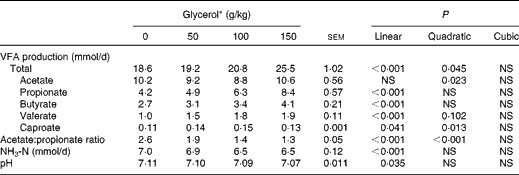
VFA, volatile fatty acids.
* Experimental substrates: 0 = 8·5 g brome hay+1·5 g maize silage; 50 = 8·5 g brome hay+1·0 g maize silage+0·5 g glycerol; 100 = 8·5 g hay+0·5 g maize silage+1·0 g glycerol; 150 = 8·5 g hay+1·5 g glycerol.
With increasing concentrations of glycerol in the substrate, 24 h cumulative gas production tended to increase linearly (P= 0·061; Table 4) and CH4 concentration in gas was linearly increased (P< 0·001). This resulted in a linear increase in CH4 production when expressed as total mg CH4/d, mg CH4/g total DM incubated (P= 0·001) and mg CH4/g of hay DM disappeared (P< 0·001).
Table 4 Effects of increasing concentrations of glycerol on cumulative gas production and methane production in the rumen simulation technique (Mean values with their standard errors)
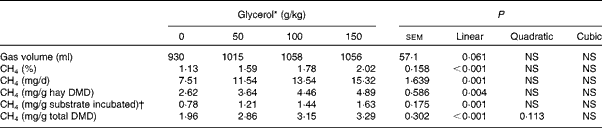
DMD, DM disappeared.
* Experimental substrates: 0 = 8·5 g brome hay+1·5 g maize silage; 50 = 8·5 g brome hay+1·0 g maize silage+0·5 g glycerol; 100 = 8·5 g hay+0·5 g maize silage+1·0 g glycerol; 150 = 8·5 g hay+1·5 g glycerol.
† DM basis.
Discussion
The effects of glycerol on fibre digestion have been variable. The linear increase in DM, NDF and ADF loss from hay and silage is in agreement with the findings of the study of Wang et al. ( Reference Wang, Lui and Huo 25 ), who reported increased in sacco effective degradability of DM and NDF from forage as well as an improved digestibility of total tract nutrients, including NDF, when steers were fed increasing concentrations of glycerol (0, 11, 22 and 33 g/kg DM) in mixed diets (600 g/kg maize stover and 400 g/kg concentrate). The results of the present study also concur with those of the study of Schröder and Südekum( Reference Schröder, Südekum, Wratten and Salisbury 26 ), who reported that fibre digestion was increased in low-starch diets when glycerol was included at a concentration of 150 g/kg DM. In another study, increasing concentrations of glycerol (0–400 g/kg diet DM) have been shown to not affect the in vitro degradability of NDF when added to lucerne hay( Reference Krueger, Anderson and Tedeschi 27 ). However, reductions in fibre digestion when glycerol is added to starch-containing diets in vivo ( Reference Schröder, Südekum, Wratten and Salisbury 26 ) and in vitro ( Reference Abo El-Nor, Abu Ghazaleh and Potu 28 ) have been reported. These results have been associated with the inhibition of hemicellulolytic and cellulolytic bacteria( Reference Abo El-Nor, Abu Ghazaleh and Potu 28 ) and fungi( Reference Roger, Fonty and Andre 29 ). Increased crude protein digestibility from silage is in contrast with the findings of previous studies, which have reported unaffected digestibility of total tract proteins in dairy cows fed glycerol( Reference Rico, Chung and Martinez 30 ) or decreased in sacco degradability of proteins in steers( Reference Wang, Lui and Huo 25 ). Discrepancies among studies on the effects of glycerol on fibre and protein digestibility are difficult to explain. It is possible that some proteolytic and fibrolytic species may have responded differently to glycerol in the present study, but this is difficult to ascertain as microbial populations were not determined. The quantification of organisms would be important to resolve contradictory results of the effects of glycerol on fibre digestion.
Previous studies have consistently reported a decreased molar proportion of acetate and increases in the proportion of propionate in in vitro conditions using glycerol in starch-rich( Reference Bergner, Kijora and Ceresnakova 9 , Reference Avila, Chaves and Hernandez 10 ) and forage substrates( Reference Avila, Chaves and Hernandez 10 , Reference Krueger, Anderson and Tedeschi 27 ), as well as in vivo in finishing beef cattle fed concentrate diets( Reference Mach, Bach and Devant 3 , Reference Schröder, Südekum, Wratten and Salisbury 26 ) and in transition dairy cows( Reference DeFrain, Hippen and Kalscheur 31 ). This concurs with the results of the present study and confirms the propiogenic properties of glycerol. Shifts towards reduced acetate:propionate ratio derived from the increased concentrations of propionate and increases in the concentrations of butyrate have also been reported in vitro using starch substrates( Reference Bergner, Kijora and Ceresnakova 9 ) and in vivo using starch- and forage-based diets( Reference Schröder, Südekum, Wratten and Salisbury 26 ).
The linear increase in CH4 proportion in total gas and total CH4 production as a function of total DM disappeared contradicts our hypothesis. The fermentation of carbohydrates to propionate has been described as a hydrogen sink, and feeding propiogenic substrates has been proposed as a CH4 abatement strategy( Reference Boadi, Benchaar and Chiquette 11 – Reference McAllister and Newbold 13 ). However, glycerol is a more reduced substrate than sugars and releases two electron pairs for each mole of glycerol converted to pyruvate( Reference Zhang and Yang 32 ), one in the oxidation of glycerol to dihydroxyacetone, which is then phosphorylated and enters glycolysis, and the other in glycolysis itself, in the oxidation of 3-phosphoglyceraldehyde to 3-phosphoglycerate( Reference Biebl, Menzel and Zeng 33 ). This compensates for electron incorporation in the conversion of pyruvate or phosphoenolpyruvate to propionate. Thus, there is no net electron incorporation in the conversion of glycerol to propionate:
.
Glycerol failed to decrease CH4 production as hypothesised, but increased it. There was an increase in butyrate production as glycerol replaced maize silage. Butyrate production from both carbohydrates and glycerol would result in a release of reducing equivalents and contribute to increasing CH4 production:
Less amounts of glycerol seem to be fermented to acetate( Reference Czerkawski and Breckenridge 34 ). Acetate production was quadratically affected by the substitution of maize silage with glycerol, but changes were of relatively low magnitude. Glycerol stimulated DM disappearance, but because glycerol replaced maize silage, the amounts of total forage digested DM were actually lower as there were less amounts of maize silage to be digested and less amounts of carbohydrates were fermented. Therefore, changes in acetate production seem to have resulted from a shift in carbohydrate fermentation towards acetate, which would also release reducing equivalents and contribute to the increase in CH4 production, because the increase in propionate production from glycerol would not demand extra reducing equivalents. The formation of some butyrate and acetate from glycerol instead of from carbohydrates would further contribute to the enhancement of methanogenesis, again because being more reduced than carbohydrates, glycerol would result in a greater release of reducing equivalents per mol of acetate and butyrate produced compared with carbohydrates.
An alternative explanation for the increase in CH4 production with glycerol is based on the equimolar conversion of glycerol to formate and ethanol by an isolate from deer rumen identified as Klebsiella planticola ( Reference Jarvis, Moore and Thiele 35 ). Formate is a precursor of CH4 ( Reference Hungate, Smith and Bauchop 36 ), and large amounts of ethanol are oxidised to acetate in the rumen( Reference Pradhan and Hemken 37 , Reference Jean-Blain, Durix and Tranchant 38 ), a process that releases reducing equivalents that can be used for CH4 production( Reference Moomaw and Hungate 39 ). It has been shown that pure cultures of Ruminococcus flavefaciens ( Reference Latham and Wolin 40 ), R. albus ( Reference Pavlostathis, Miller and Wolin 41 ) and a ruminal fungus( Reference Bauchop and Monfort 42 ) decrease formate and ethanol production when co-cultured with methanogens, as CH4 becomes the main electron sink in the co-cultures. Also, some micro-organisms can convert glycerol to 1,2-propanediol( Reference Clomburg and Gonzalez 43 ), and in turn there is some recovery of 1-14C-1, 2-propanediol incubated in ruminal continuous cultures as 14CH4 ( Reference Czerkawski, Piatkova and Brekenridge 44 ).
The adaptation of donor animals to diets containing glycerol seems to have affected fermentation when glycerol was included in in vitro batch culture incubations. Gas and CH4 production was increased when 150 g/kg glycerol was included in the substrates (900 g/kg concentrate based on rolled maize, maize gluten feed and soyabean hulls) using inoculum obtained from glycerol-adapted animals( Reference van Cleef, Uwituze and Drouillard 45 ), but changes in CH4 production were negligible when the inoculum was obtained from unadapted animals, suggesting that microbial adaptation influences digestion and fermentation end products. This explains, at least partially, the differences between previous studies reporting no effect( Reference Avila, Chaves and Hernandez 10 ) or decreased CH4 production( Reference Lee, Lee and Cho 14 ) when incubating glycerol in in vitro batch cultures using inoculum obtained from unadapted animals as opposed to the results of the present study, where increased propionate and total VFA production and linear increase in DM loss were found to be associated with increased CH4 production (mg CH4/g DM digested) using glycerol-adapted fermenters. When increasing concentrations of glycerol were fed to adapted lambs, no effects on CH4 emissions were observed( Reference Avila-Stagno, Chaves and He 4 ). In this case, absorption through the rumen wall or passage to the lower gut or both may have impeded fermentation of an important proportion of glycerol( Reference Rémond, Souday and Jouany 7 ), thus reducing the release of hydrogen electrons in the rumen environment when compared with in vitro fermenters where absorption is precluded.
Conclusions
Increasing concentrations of glycerol in forage diets incubated in a RUSITEC apparatus improved DM, NDF and ADF disappearance from brome hay and maize silage and crude protein disappearance from maize silage. The acetate:propionate ratio was linearly decreased as a result of increased production of propionate. The concentrations of CH4 in gas and total CH4 production per unit of DM digested or incubated were increased, as the fermentation of glycerol to propionate does not act as a hydrogen sink.
Acknowledgements
The present study was supported by Canada–Norway Greenhouse Gas Project and the SAGES programme of Agriculture and Agri-Food Canada. J. A.-S. is supported by a Conicyt-Chile Scholarship. The funding agencies had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions were as follows: J. A.-S., A. V. C. and T. A. M. designed the study; J. A.-S. and G. d. O. R. conducted the experimental procedures and laboratory analyses; J. A.-S. and A. V. C. analysed and interpreted the data; J. A.-S. wrote the first draft of the manuscript; A. V. C., T. A. M., E. M. U. and G. d. O. R. critically revised the manuscript.
The authors declare that there is no conflict of interest with any financial organisation regarding the material discussed in the manuscript.








