Ungulate populations worldwide whose survival depends on the ability to embark on long distance migrations or make large scale nomadic movements are faring poorly (Berger, Reference Berger2004; Wilcove, Reference Wilcove2008). In the 19th century North American bison Bison bison were all but eliminated as a result of railway-facilitated accessibility for hunters (Isenberg, Reference Isenberg2000), and in the 20th century wildebeest Connochaetes taurinus in Botswana were severely depleted by veterinary cordon fences (Spinage et al., Reference Spinage, Williamston, Williamston and East1989, cited in Nowak, Reference Nowak1991). More recently, saiga antelope Saiga tatarica declined from over one million animals to near extinction in less than 10 years because of intense hunting (Milner-Gulland et al., Reference Milner-Gulland, Bekenev and Grachov1995, Reference Milner-Gulland, Bukereeva, Bekenov and Grachev2005; Sharp, Reference Sharp2002), as have chiru Pantholops hodgsoni in and near the Chang Tang Nature Reserve on the Tibetan Plateau (Schaller, Reference Schaller2002; Schaller et al., Reference Schaller, Kang, Gai and Yanlin2006). Car-ibou Rangifer tarandus in the USA and Canada are vulnerable to oil development activities (Joly et al., Reference Joly, Nellemann and Vistnes2006). Some species have been protected by means of large refuges, such as wildebeest in the Serengeti-Mara ecosystem (Thirgood et al., Reference Thirgood, Mosser, Tham, Hopcraft, Mwangomo and Mlengeya2004) but because of the nature of their large scale movements, far-ranging ungulates are difficult to conserve.
Mongolian gazelles Procapra gutturosa face an uncertain fate. With their demise, the world would lose not just another species but also a spectacular wildlife phenomenon that the explorer Roy Chapman Andrews (Reference Andrews1932) once identified as a rival of the other great and better known African mammal assemblages. More recently the Mongolian gazelles and their steppe habitat have been described as one of the largest remaining intact grazing systems in the world (Schaller, Reference Schaller1998).
Here we report the observation of one of the largest ungulate aggregations (> 200,000) recorded in Eurasia: a mega-herd of Mongolian gazelles. We relate our observation to existing habitat models of this species and describe how these habitats are potentially being affected. Finally, we discuss the consequences of habitat loss for the nomadic, large-scale movements exhibited by Mongolian gazelles.
Mongolian gazelles are one of the last abundant, nomadic ungulates. A commonly repeated anecdote by local inhabitants is that Mongolian gazelles used to be seen in groups large enough to give the appearance that the ground before them was moving. Although herds of > 10,000 are commonly reported (Sokolov & Lushchekina, Reference Sokolov and Lushchekina1997), the largest herds historically reported numbered c. 80,000 (cited in Lhagvasuren & Milner-Gulland, Reference Lhagvasuren and Milner-Gulland1997). Enduring decades of intense hunting, the population of Mongolian gazelles has declined significantly and was recently categorized as regionally Endangered (IUCN, 2003; Clark & Javzansuren, Reference Clark, Javzansuren, Dulamtseren, Baillie, Batsaikhan, Samiya and Stubbe2006). Habitat loss and fragmentation throughout the grasslands are additional significant threats to the species’ survival (Gunin et al., Reference Gunin, Vostokova, Dorofeyuk, Tarasov, Black, Gunin, Vostokova, Dorofeyuk, Tarasov and Black1999; Humphrey & Sneath, Reference Humphrey and Sneath1999). The eastern steppe of Mongolia, a 250,000 km2 area and the last stronghold for Mongolian gazelles, represents only a fraction of what was once a 1.5 million km2 temperate grazing ecosystem (Tong et al., Reference Tong, Wu, Yong, Yang and Yong2004). The remaining critical habitat faces further degradation from oil exploitation and road and railroad development.
During 3–14 September 2007 we conducted field work in Eastern Mongolia, capturing and radio-collaring nine adult female Mongolian gazelles as part of a long-term study of the species’ movement ecology. Our capture locations were ‘islands’ of green vegetation with abundant gazelles (Fig. 1, Location A) surrounded by vast areas of uninhabited, drought-stricken steppe. Gazelle herds of a few dozen individuals to one herd numbering 6,000–8,000, an unusual sighting in itself, were seen grazing in the greened areas.

Fig. 1 The eastern steppe of Mongolia where observations took place. The background image displays probability of gazelle occurrence using a model developed by Mueller et al. (Reference Mueller, Olson, Fuller, Schaller, Murray and Leimgruber2008) and applied to satellite imagery of vegetation productivity (NDVI) of the survey period. Lettered locations are for reference with the text. The inset indicates the location of the main figure in eastern Mongolia.
On 11 September, after completing the collaring, we travelled to south-eastern Dornod Aimag in search of more gazelles and to explore other habitats in the Eastern steppes. In the following days we drove a total of c. 750 km through an area of c. 30,000 km2. Initially, we crossed a drought-stricken region with virtually no gazelles (Fig. 1, B). As we left this area the vegetation greened considerably and we again encountered herds ranging from several hundred to thousands of animals (Fig. 1, C).
On 12 September we encountered the largest aggregation of Mongolian gazelles ever recorded (Fig. 1, D). It appeared that these gazelles had been feeding heavily on lush green leaves of Cirsium spp. and most plants in the vicinity showed evidence of freshly bitten leaves. Fresh moist dung and urine abounded. From a prominent (50 m) hilltop offering a nearly 360° view, herds of Mongolian gazelles were visible nearly everywhere within a 10–20 km radius (although no actual measurement of this distance was recorded, and thus no error term calculated).
The size and extent of this mega-herd was estimated, by point observation, independently by P. Leimgruber and K. Olson, both of whom have experience estimating herd sizes (Olson et al., Reference Olson, Fuller, Schaller, Odonkhuu and Murray2005a). Initially, individuals in groups of 10–100 were counted, groups of 100 aggregated to identify groups of 1,000, and sometimes groups of 1,000 counted to estimate a group of 10,000 (cf. North-Griffiths, Reference Norton-Griffiths1978). The number of groups of 100, 1,000 or 10,000 surrounding the hilltop were counted and totalled several times by both observers (Plate 1), and their overall best estimates were 200,000–250,000 animals. All estimates were repeated many times but such total counts almost always provide underestimates (Norton-Griffiths, Reference Norton-Griffiths1978; Krebs, Reference Krebs and Sutherland2006).

Plate 1 Groups of Mongolian gazelles making up part of an aggregated herd of 200,000–250,000 seen in the eastern steppe of Mongolia on 12 September 2007 (Fig. 1). In the foreground, c. 325 gazelles can be seen. In the distance c. 1,100 are visible, and not visible on the far distant grasslands are thousands more.
By estimating that the visible aggregation covered an area of c. 800 km2, the density was probably 250–312 km-2. We also suspect that this herd extended well beyond the visible horizon because as we subsequently continued moving east, we watched large gazelle groups crossing in front of our vehicle for an additional 4 hours. Hence, this group could have been > 250,000. Given the most recent estimate (2005) of 1.13 million gazelles occurring throughout the entire range of gazelles in Mongolia east of the Ulaanbaatar-Beijing railroad, where nearly all of this species resides (Olson, Reference Olson2008), this mega-herd represented at least 18% of the total population of the species.
Continuing our survey, we passed through an area characterized by low, wind-blown dunes vegetated with Salix spp. and Ulmus chinensis, scattered fresh water ponds and springs, and abandoned agricultural land (Fig. 1, E). Within this section we observed no gazelles but encountered intense harassment by mosquitoes. On the last day of our survey we crossed through an area of lush green Stipa grassland that is now undergoing intensive development of oil deposits (Fig. 1, F). In this area we observed only a few scattered groups of gazelles totalling < 100 individuals.
Most gazelle herds seen in previous autumns were much smaller (Olson et al., Reference Olson, Fuller, Schaller, Odonkhuu and Murray2005a). During late September–early October 2001 and late August–early September 2002, long-distance (1,356 and 1,186 km, respectively) line transect surveys were driven across all of the area that we drove in September 2007. The total of 17,267 and 23,429 gazelles seen in 125 and 110 herds, respectively, were in herds of 1–2,500, averaging 138 and 213 animals in each herd, respectively (median herd size 42 and 14, respectively).
Understanding the factors contributing to unusually large gazelle aggregations and the implications for gazelle ecology are important for conservation of the species. Telemetry data concerning gazelle movements (Olson, Reference Olson2008) indicated that individuals captured at the same site subsequently use completely different areas in the same year, and that individual animals may use ranges of up to 25,000 km2 per year. Also, ranges of individuals are not geographically predictable among years, probably as a result of changing and unpredictable distribution of preferred habitats (Mueller et al., Reference Mueller, Olson, Fuller, Schaller, Murray and Leimgruber2008). Thus, in most years, gazelles are spread widely throughout the steppe (Olson, Reference Olson2008) in relatively small groups.
To identify how the mega-herd formed we used a previously developed second-order logistic model to predict gazelle occurrence based on satellite-based estimates of relative vegetation productivity (NDVI; Mueller et al., Reference Mueller, Olson, Fuller, Schaller, Murray and Leimgruber2008). The model was derived by quantifying the distribution of gazelles during two springs and two autumns in the same area where our mega-herd and related observations occurred (Olson et al., Reference Olson, Fuller, Schaller, Odonkhuu and Murray2005a), and tested with independent data from a gazelle telemetry study (Olson et al., Reference Olson, Fuller, Schaller, Lhagvasuren and Odonkhuu2005b). Mueller et al. (Reference Mueller, Olson, Fuller, Schaller, Murray and Leimgruber2008) found that gazelles preferred an intermediate range of NDVI, which are presumably those areas that provide sufficient quantity of forage with a high digestibility (i.e. high N content). We applied this model retrospectively to satellite data (MODIS-NDVI 16-day composite) from the first 2 weeks of September 2007 (when we made our observations) to test whether the model could accurately predict the suitability of the location of the observed mega-herd. Both the location of the mega-herd and the capture locations where a substantial concentration of gazelles was found were predicted as high-preference areas by the model (Fig. 1). Similarly, the intervening areas where we observed no or few gazelles corresponded with areas of low vegetation productivity and were predicted by the model to harbour few gazelles. Exceptions to the model predictions were the regions affected by mosquito abundance and those with oil production activities, where despite predicted high quality habitat virtually no gazelles were observed. Presumably, insect and human disturbance, respectively, prevented gazelles from using these areas.
This unusual and high concentration of gazelles is, at first consideration, a biological wonder and indicative of a species whose numbers have not yet been decimated by poaching or other causes. However, locations of good gazelle habitat vary considerably both within and among years depending on precipitation and drought conditions (Yu et al., Reference Yu, Price, Ellis, Feddema and Shi2004; Mueller et al., Reference Mueller, Olson, Fuller, Schaller, Murray and Leimgruber2008). For example, during summer 2007 precipitation in some areas was well below average but in areas near sites predicted to be high quality gazelle habitat in September, summer rains, especially those in August, were unusually heavy (Fig. 2). Given this spatial and temporal unpredictability in habitat quality, the majority of suitable gazelle habitat in the region may infrequently, but critically, be restricted to areas affected either by insects, oil field development, or other human disturbances such as roads, railways (Ito et al., Reference Ito, Miura, Lhagvasuren, Enkhbileg, Takatsuki, Tsunekawa and Jiang2005) or agriculture (Wang et al., Reference Wang, Helin, Junghui and Ming1997). We do not know the frequency with which such range restriction occurs, and our unique observation suggests it is rare but not so rare that it should be excluded from conservation consideration. Historically, gazelles have probably been able to persist, despite insects, because the area over which they could possibly range was so large. But with new and expanding human activities, the refuges available to gazelles in some years may not be available and, consequently, the gazelles could suffer decreased fecundity and/or direct mortality from starvation. Given the extent and unpredictability of the gazelles’ movements, and the potential for infrequent but major range restrictions, current protected areas (Fig. 1) seem inadequate.
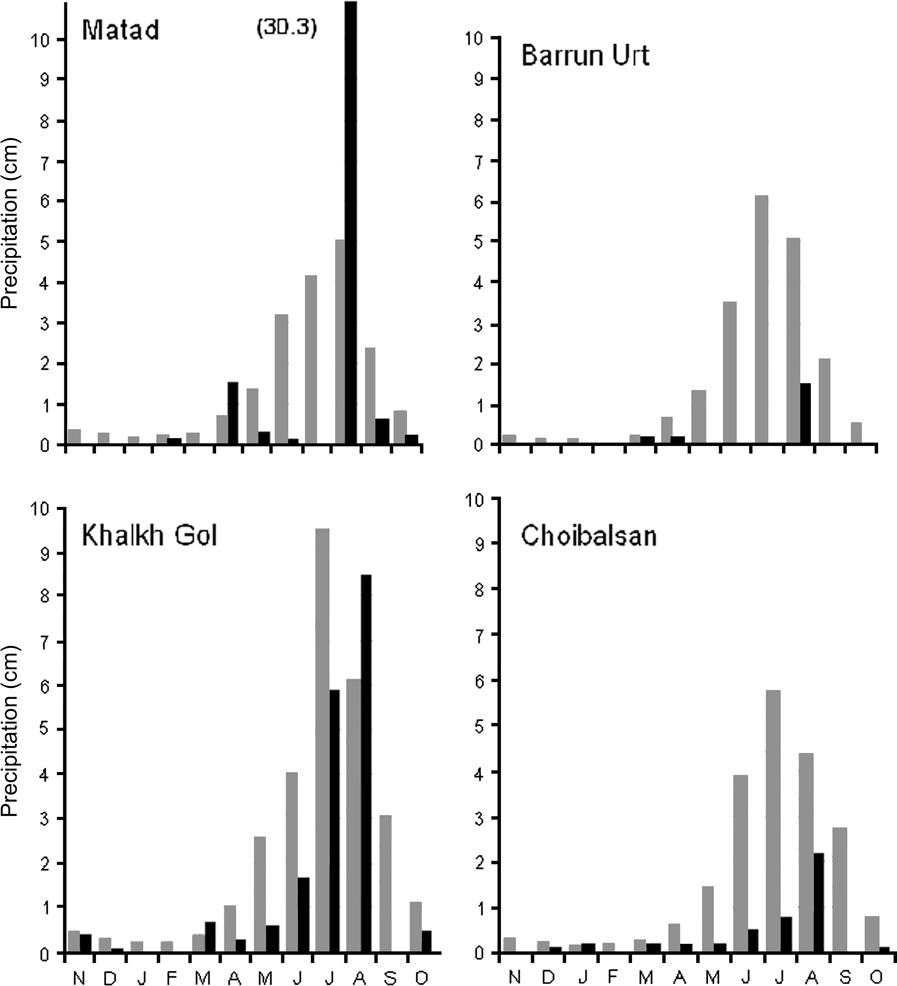
Fig. 2 Seasonal and historical variation in precipitation at four weather stations within the survey region (from Weather Underground, 2008: see Fig. 1 for locations). Black bars are precipitation over November 2006–October 2007; grey bars are historical averages (c. 30 years). Matad received 30.3 cm of rain during 1 day in late August.
Evidence suggests that the Mongolian steppes are at a crossroads, and with them their abundant wildlife populations (Goyal, Reference Goyal1999; Reading et al., Reference Reading, Bedunah, Amgalanbaatar, Bedunah, McArthur and Fernandez-Gimenez2006). Efforts are underway to bring economic development to the region via oil and mineral extraction and the development of transport infrastructure. Ongoing changes to Mongolia's land ownership laws will grant exclusive long-term rights to pasture land to private companies and collectively organized households. Without a well-organized voice on behalf of the ecology of the steppe, this vast grassland and the great herds of Mongolian gazelles will also probably be reduced. Developing landscape-level strategies pertaining to the entire steppe that allow access to all the grasslands as needed, rather than designating a single or a small handful of protected areas, will be key to conserving Mongolian gazelles effectively.
Acknowledgements
Funding was provided in part by grants from the National Science Foundation (DEB-0608224, DEB-0743557, DEB-0743385), and a Smithsonian Predoctoral Fellowship. The Wildlife Conservation Society and USAID provided in-country financial and logistical support. The University of Maryland and University of Massachusetts provided support for WFF and TKF. The Mongolian Ministry of Nature and Environment and Dornod Aimag Environmental Inspection Agency granted permission to carry out this research. Reviews by J. Berger and an anonymous referee were extremely helpful.
Biographical sketches
Kirk A. Olson is incorporating results from field studies of Mongolian gazelles into conservation strategies. Thomas Mueller uses remote sensing and animal movement data to develop habitat use and prediction models that can be used for the conservation of critically important habitat. Sanjaa Bolortsetseg conducts field research and works with rural households in eastern Mongolia to promote community-based conservation efforts targeting declining wildlife populations. Peter Leimgruber incorporates geographical information systems and satellite tracking to develop conservation and management strategies for threatened charismatic species. William F. Fagan meshes field research and theoretical models to develop quantitative approaches to help solve real-world problems in conservation biology. Todd K. Fuller focuses on identifying factors affecting variation in mammal density and distribution to manage population change responsibly.







