Article contents
Migration of point defects and a defect pair in zinc oxide using the dimer method
Published online by Cambridge University Press: 25 May 2012
Abstract
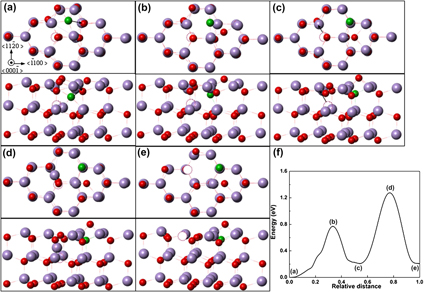
The migration mechanism and the minimum energy path of vacancies, interstitials, and an interstitial–vacancy pair in zinc oxide have been studied by the dimer method. The in-plane and out-of-plane migrations of zinc and oxygen vacancies are anisotropic. The kick-out mechanism is energetically preferred to zinc and oxygen interstitials that can easily migrate through the ZnO crystal lattice. In addition, the migration process of an interstitial–vacancy pair as a complex of an octahedral oxygen interstitial and a zinc vacancy is dominated by an oxygen interstitial/zinc vacancy successive migration. The energy barriers indicate that the existence of oxygen interstitial in the defect pair can promote the mobility of zinc vacancy, whereas the migration of oxygen interstitial is slowed down due to the presence of zinc vacancy. In the end, we show a possible migration path of the interstitial–vacancy pair that can be dissociated through a set of displacement movements.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 27 , Issue 17: Focus Issue: Oxide Semiconductors , 14 September 2012 , pp. 2241 - 2248
- Copyright
- Copyright © Materials Research Society 2012
References
REFERENCES
- 7
- Cited by




