INTRODUCTION
Vibrio are Gram-negative, rod-shaped bacteria commonly found in warm coastal waters worldwide [Reference Morris1, Reference Tantillo2]. The Centers for Disease Control and Prevention (CDC) estimates that Vibrio spp. cause over 8000 infections annually [Reference Mead3], and the incidence of vibriosis has increased for all species since 1996 [Reference Voetsch4, Reference Vugia5], due in part to better surveillance and reporting. In the USA, most cases of vibriosis are reported from Gulf Coast states, where vibrios are commonly found in their natural habitat [Reference Strom and Paranjpye6–Reference Dechet8]. Infections are acquired by consuming contaminated food or water or exposing wounds and abrasions to marine environments [Reference Morris1, Reference Tantillo2]. Cases typically occur in the warm summer months.
The more common species in the USA include Vibrio vulnificus and V. parahaemolyticus. V. vulnificus is often associated with raw oyster consumption, wound infections, and primary septicaemia [Reference Tantillo2]. V. parahaemolyticus is typically associated with gastroenteritis. Other common species include V. cholerae non-O1 (gastroenteritis) and V. alginolyticus (wound infections) [Reference Morris1].
Vibriosis has the highest case-fatality rate (CFR) of any enteric disease, mainly attributable to V. vulnificus, making this a very important public health concern despite the low incidence reported annually. Based on FoodNet data from 2007, the incidence of vibriosis was 0·2 cases/100 000 individuals, and the CFR was 3·6% [9]. Incidence rates for other common foodborne bacterial enteric diseases in the USA are higher, although CFRs are much lower. Based on these same data, rates for the most common foodborne diseases are (incidence per 100 000, case fatality): Salmonella (14·9, 0·4%), Campylobacter (12·8, 0·1%), Shigella (6·2, 0·1%), and Escherichia coli O157:H7 (1·2, 0·2%) [9]. The highest overall CFR for common foodborne enteric diseases (Salmonella, 0·4%) is almost 90% less than the overall CFR for vibriosis (3·6%). Historically, Florida's incidence rate for vibriosis (0·4/100 000) is double and the CFR (10·0%) is almost triple the national rate [Reference Hlady and Klontz10].
Because of the high CFRs associated with V. vulnificus from consumption of raw oysters, there has been a focus in the Gulf Coast states to increase awareness in high-risk individuals, i.e. those with underlying health conditions. As required by Florida law since 1993, all food establishments selling raw oysters must have a visible warning posted to make the consumer aware of health risks associated with their consumption. The Florida Department of Health (FDOH) also regularly distributes educational materials and presents data and risk reduction messages at venues statewide to a variety of audiences, from health professionals to consumers. The goal of these efforts is to increase awareness in high-risk population groups and healthcare providers in order to reduce incidence and case fatality. The vast majority of the current messaging is related to the risk of infection with V. vulnificus associated with raw oyster consumption.
Vibriosis has been a notifiable disease in Florida since 1981, in the Gulf Coast states since 1988, and nationally since 2007. However, most states have been voluntarily reporting since 1997. The epidemiology of vibrio infections in Florida has been described previously [Reference Hlady and Klontz10, Reference Hlady, Mullen and Hopkins11]. From 1981 to 1993, the annual incidence of vibriosis was 4·3/1 000 000 individuals. The most common species reported during this period were V. parahaemolyticus, V. vulnificus, and V. cholerae non-O1, respectively, with gastroenteritis being the most commonly reported clinical syndrome, with raw oyster consumption in the week prior to illness reported in 45% of cases [Reference Hlady and Klontz10]. To determine whether trends in vibriosis have changed with time, we sought to describe the current epidemiology of vibrio infections in Florida using data collected from 1998 to 2007.
METHODS
We examined cases of vibriosis reported to FDOH with onset dates from 1998 to 2007. Data were collected using the ‘Cholera and Other Vibrio Illness Surveillance Report’ (CDC Form 52.79). The average annual incidence rate for cases of vibriosis was calculated using yearly population data from Florida Charts [12] based on data from the Florida Legislature, Office of Economic and Demographic Research.
Descriptive analyses were performed for all reported cases and examined by species. Medians (ranges) were reported for non-normally distributed continuous variables and frequencies (percentages) for categorical variables. Kruskal–Wallis tests were used to compare median values. Crude odds ratios (ORs) and 95% confidence intervals (CIs) were reported for bivariate analyses.
Logistic regression was used to assess the association between various demographic and disease characteristics and death from vibriosis. Predictor variables that were considered in our analyses included age, race/ethnicity, gender, clinical syndrome, exposure, and presence and numbers of underlying health conditions. The clinical syndrome field included the following categories: septicaemia, the presence of bacteria in the blood which can be characterized by fever and chills to hypotension and shock; wound infection, either from injuries sustained in aquatic environments or from pre-existing wounds and characterized by fever, cellulitis, and pain around the site of infection; and gastroenteritis, an inflammation of the stomach and intestines characterized by diarrhoea and vomiting. Adjusted ORs and 95% CIs were reported. Data were analysed using SAS version 9.1 (SAS Institute, USA).
RESULTS
All species
There were 834 cases of vibriosis in 825 individuals reported to FDOH from 1998 to 2007 (median 82·5/year). The average annual incidence rate was 4·8 cases/1 000 000 individuals. Infections were most common in males (71·8%), whites (84·5%), and non-Hispanics (84·9%). The median age of reported cases was 50·0 years (range 0–96 years), with the majority (74·9%) between the ages of 30 and 79 years. Seven individuals were simultaneously infected with multiple species.
The most common species were V. vulnificus, V. parahaemolyticus, and V. alginolyticus (Table 1). The frequency of isolated species varied by year of diagnosis (Fig. 1), with most cases occurring during months when the water temperature and weather are warmer (Fig. 2). Seasonality did not vary by species; however, age at diagnosis was different (P<0·0001). The median age at diagnosis for cases of V. vulnificus was 58 years, compared to 36 years in cases of V. alginolyticus and 42 years for V. parahaemolyticus.
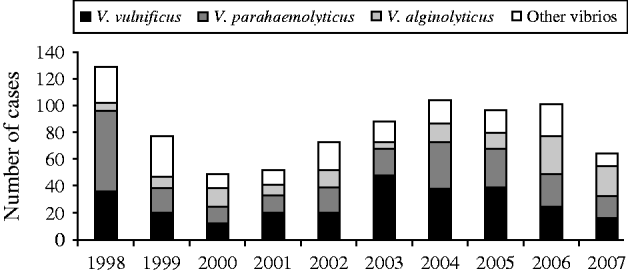
Fig. 1. Cases of vibriosis by species and year of diagnosis, Florida, 1998–2007.
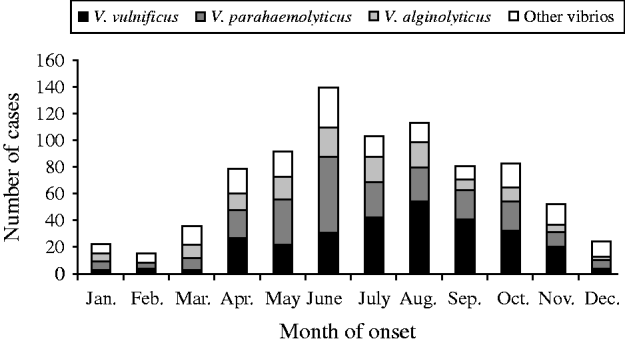
Fig. 2. Seasonality of vibriosis by species, Florida, 1998–2007.
Table 1. Number of reported cases of vibriosis by species, Florida, 1998–2007

* Seven individuals were infected with multiple Vibrio species.
Wound-related symptoms were reported for 373 (44·7%) cases, followed by 350 (42·0%) with gastroenteritis, 45 (5·4%) with septicaemia, and 66 (7·9%) with other reported symptoms (Fig. 3). There were 220 (26·4%) cases attributed to raw oyster consumption, 313 (37·5%) to wound infections, 96 (11·5%) to other seafood exposures, and 205 (24·6%) had no reported exposure (Fig. 4).
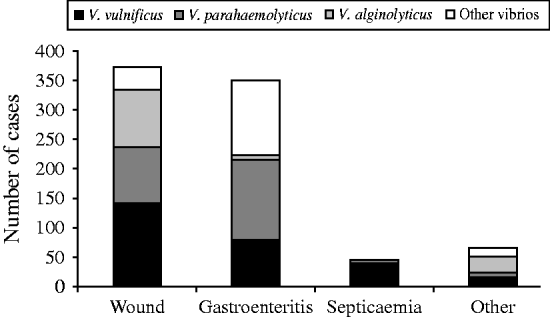
Fig. 3. Clinical syndromes of vibriosis by species, Florida, 1998–2007.
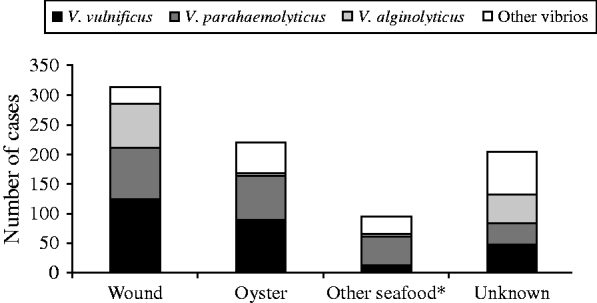
Fig. 4. Exposures associated with cases of vibriosis by species, Florida, 1988–2007. * Other seafood includes clams, mussels, shrimp, crab, fish, etc.
Presence of any underlying health condition was reported for 400 (48·5%) cases. Of these, 228 (57·0%) had multiple conditions reported. Heart disease, alcoholism, and liver disease were the most common (Table 2).
Table 2. Underlying conditions in cases of vibriosis, Florida, 1998–2007

There were 82 deaths reported, yielding a CFR of 9·9%. Age at onset, gender, presence of any underlying health condition and total number of conditions, mode of exposure, and clinical syndrome were significantly associated with death. The odds of death from vibriosis increased by 2·3% for every 1 year increase in age (P=0·0001). Median age at diagnosis was higher (P=0·0001) for cases resulting in death than those that did not. Being male was associated with death (crude OR 2·92, 95% CI 1·48–5·78). Most deaths (95·1%) had one or more underlying health conditions reported. The odds of death in cases with one or more underlying health conditions were 25·5 (95% CI 9·2–70·4) times that of cases with no underlying condition. The odds increased with the total number of underlying health conditions (P<0·0001). Odds of death also differed by exposure and clinical symptoms, with oyster consumption, gastroenteritis, and septicaemia being significantly associated with death (Table 3).
Table 3. Predictors of death in cases of vibriosis, Florida, 1998–2007

OR, Odds ratio; CI, confidence interval.
* Compared to no underlying condition.
† Excludes cases with unknown exposure (n=203).
In multivariable analyses, the best predictive model for death from vibriosis included total number of underlying health conditions and exposure, based on model fit statistics. The odds of death increased with increasing number of underlying conditions: one (OR 9·8, 95% CI 2·7–35·6), two (OR 18·7, 95% CI 5·3–66·3), and three or more conditions (OR 50·7, 95% CI 14·5–177·1). The odds of death were greater for exposure through oyster consumption (OR 6·7, 95% CI 3·2–14·0) compared to wound infection.
V. vulnificus
From 1998 to 2007, there were 276 cases of V. vulnificus reported (average annual incidence 1·6/1 000 000). There were 141 (50·7%) cases presenting with wound-related symptoms, 79 (28·6%) cases presenting with gastroenteritis, 40 (14·5%) with septicaemia, and 16 (5·8%) with unknown syndrome reported (Fig. 3). The most common cause was wound infection (125 cases, 45·3%). Ninety-one (33·0%) cases were associated with oyster consumption, 12 (4·3%) with other seafood consumption, and 48 (17·4%) had unknown cause (Fig. 4). At least one underlying health condition was reported for 75·0% of cases, with most (70·0%) reporting multiple conditions.
There were 76 deaths among V. vulnificus infections (CFR 27·5%), with 33 deaths in those presenting with septicaemia (CFR 82·5%), and 73 in those with underlying health conditions (CFR 96·1%). Most (56%) deaths were associated with raw oyster consumption, and 14% were associated with wound infections. The odds of death in cases of V. vulnificus with one or more underlying conditions were 11·8 (95% CI 3·6–38·8) times the odds of death in those with none.
V. parahaemolyticus
There were 245 cases of V. parahaemolyticus reported (average annual incidence 1·4/1 000 000). The most common presenting clinical syndromes were gastroenteritis (137 cases, 55·9%) and wound infection (96 cases, 39·2%) (Fig. 3). Most (50·2%) cases were associated with consumption of seafood: 73 (29·8%) with oysters and 50 (20·4%) with other seafood (e.g. crabs, shrimp). There were 86 (35·1%) cases associated with wound infections (Fig. 4). Eighty-four (32·6%) cases had an underlying health condition reported. There was one death in cases of V. parahaemolyticus infection.
From 1998 to 2007, there were 28 documented outbreaks of V. parahaemolyticus that included 301 individuals [average 11 cases (range 2–115)]. Of the outbreaks, 35·7% were laboratory confirmed. Most (82·1%) were associated with seafood consumption at a restaurant. Crustacean shellfish were the most commonly implicated source (50·0%), followed by molluscan shellfish (28·6%), with specific vehicles including shrimp (28·6%), crabs (25·0%), and oysters (21·4%). The most frequent contamination factors were cross-contamination from raw ingredients of animal origin, bare-handed contact, and inadequate cleaning. Almost all of the reported proliferation factors were related to time-temperature abuse, including inadequate cold-holding and slow cooling.
V. alginolyticus
There were 131 cases of V. alginolyticus reported (average annual incidence 0·7/1 000 000). Most cases presented with wound infections (97 cases, 74·1%) or other symptoms (27 cases, 20·6%) (Fig. 3), and most were associated with exposure from wounds (74 cases, 56·5%) (Fig. 4). All cases in individuals aged 0–19 years presented with wound or other symptoms. Forty-one (31·3%) cases had at least one underlying health condition. There were no outbreaks and no deaths associated with V. alginolyticus during this 10-year period.
DISCUSSION
This analysis updates a previous summary of reported vibrio infections in Florida [Reference Hlady and Klontz10]. The clinical and epidemiological features of vibriosis have changed since the 1996 report. Most notably, we have seen a change in common species and risk factors and an increase in incidence and CFRs. The most commonly reported species changed from V. parahaemolyticus (1981–1993) to V. vulnificus (1998–2007). Cases of vibriosis attributed to raw oyster consumption have decreased from 45% to 26% over the same time-frame. Wound infections were the most common clinical syndrome in the current analysis compared to gastroenteritis as reported by Hlady et al. [Reference Hlady and Klontz10]. Incidence of vibriosis has increased in Florida from 4·3/1 000 000 individuals (1981–1993) to 4·8/1 000 000 (1998–2007). CFRs also increased for vibriosis presenting with gastroenteritis (2–9%) and septicaemia (47–76%). Due to changes in the way risk factor and background population data were recorded over the years, we were unable to assess the relative risks in certain high-risk groups, such as people with AIDS and raw oyster-consuming adults, as done previously [Reference Hlady and Klontz10].
V. vulnificus was the most common species in Florida, unlike other areas of the country where V. parahaemolyticus was the most common [13]. V. vulnificus is associated with more serious illness [Reference Morris1, Reference Voetsch4, Reference Bross7], and is the leading cause of death related to seafood consumption [Reference Strom and Paranjpye6, Reference Bross7]. V. parahaemolyticus is recognized as the leading cause of gastroenteritis associated with seafood consumption in the USA [Reference Yeung and Boor14–16], and is often associated with foodborne outbreaks [Reference Morris1, Reference Yeung and Boor14, Reference Daniels17]. The median number of cases reported annually in Florida has increased from 16 (1981–1993) [Reference Hlady and Klontz10] to 20 cases (1998–2007). An increase in incidence of V. parahaemolyticus has also been noted throughout the USA and in other countries [Reference Morris1, Reference Yeung and Boor14]. Rising water temperatures [Reference Morris1, Reference Daniels17] and the emergence of new strains of V. parahaemolyticus throughout the world, specifically the O3:K6 strain and its serovariants [Reference Nair18] have been suggested as possible causes. Finally, V. alginolyticus is commonly associated with ear infections and illness in younger individuals [Reference Dechet8], with similar associations found in Florida.
Wound infections and seafood consumption were an equal source of exposure to Vibrio spp. in Florida, whereas seafood consumption is most often associated with vibriosis throughout the USA [13]. A possible cause for increased wound-related exposures is that Florida has over 2000 miles of shoreline [19]. This access may lead to increased participation in water activities, possibly yielding a higher number of wound exposures. Further, Florida has higher average ambient air and water temperatures year-round than many parts of the country, which may increase the likelihood of such exposures because of the longer season for water activities and higher vibrio bacterial counts.
In accord with other studies, incidence and mortality rates associated with vibriosis in Florida were higher in summer months [Reference Voetsch4, Reference Daniels17] and in males [Reference Morris1, Reference Dechet8], consistent with historical Florida trends [Reference Hlady and Klontz10]. Male gender was significantly associated with death in crude analysis; however, gender was not a significant predictor or confounder in adjusted analyses. Another important feature of vibriosis is that the greatest risk of illness and death is in those with underlying health conditions. We also found a strong association between underlying health conditions and death from V. vulnificus. Individuals with alcoholism and liver or heart disease have increased risk of infection, as well as increased risk of death.
Increases in incidence and CFRs seen in Florida may be partially attributable to increased awareness of vibriosis in healthcare providers and increased surveillance. In addition, we have experienced several large outbreaks of V. parahaemolyticus in Florida during this period. However, increases in incidence have also been found in FoodNet states [Reference Voetsch4]. Even after excluding cases of V. parahaemolyticus that occurred during known outbreaks, incidence in this species in the FoodNet states has also increased [Reference Voetsch4].
It is alarming that there are higher incidence and fatality rates in Florida despite increased educational campaigns aimed at high-risk groups. Other Gulf Coast states have seen similar increases in incidence over time, as well. Louisiana had an average annual incidence rate in 2000–2001 of 5·9 cases/1 000 000 compared to 6·7 in 2006–2007, while Mississippi increased from 2·3 to 2·9/1 000 000 and Texas increased from 1·4 to 2·3/1 000 000 during those same years [20].
Florida law requires a warning notice in all food service establishments and retail markets serving or selling raw oysters. The FDOH interacts with statewide medical organizations and health support groups to present prevention messages to the healthcare community, and distributes educational pamphlets and materials at health fairs. Further, there are several ongoing national educational campaigns sponsored by the Interstate Shellfish Sanitation Conference (ISSC) [21] and the Gulf & South Atlantic Fisheries Foundation [22] that aim to raise awareness in people with underlying health conditions regarding their risk of V. vulnificus infection associated with raw oyster consumption [23]. Given the increasing incidence seen nationwide, the ISSC is currently also working on regulatory policies related to molluscan shellfish in order to prevent vibriosis.
Florida education efforts seem to have been successful, with an almost 50% reduction in total number of cases associated with raw oyster consumption compared to that reported previously [Reference Hlady and Klontz10]. Despite this success, these campaigns are mainly funded to focus on one high-risk group, raw oyster consumers, and exclude those with wound infections. Florida has seen an increase in the number of cases associated with wound infections. While there is a continued need to warn consumers about the risk from consuming raw oysters, future prevention efforts should focus on those at highest risk for wound infections such as those with diabetes. Outreach efforts should target specialist groups for wounds such as wound care treatment centres, endocrinologists, and other providers that treat diabetic patients, as well as local diabetes support groups. These new educational campaigns should include risk messaging related to wounds sustained during water-related recreational activities or exposure to aquatic environments. Outreach programmes should focus on V. vulnificus-related wound infections. Although it is possible to get wound infections from other vibrios, V. vulnificus is particularly virulent, and messaging should focus on the recognition, treatment, and prevention of these infections.
Little research has focused specifically on wound-related cases of vibriosis which are more numerous than oyster-related V. vulnificus infections; therefore, another direction would be to examine the extent of wound infections and treatment of these cases, including wound debridement and amputation. Research is also needed to address the potential impact that the environment has on increasing incidence of vibriosis, particularly the role that environmental conditions in oyster harvesting areas have on the bacterial count of harvested oysters.
There are a few limitations that should be noted. We chose to examine the 10 most recent years with complete vibrio data available. The previous study included data up to 1993 [Reference Hlady and Klontz10]. However, we did not include the years 1994–1997 in our analysis due to concerns regarding data quality for those years. As with all foodborne illnesses, cases of vibriosis are underreported, and reporting is probably biased towards more severe cases. Cases of non-cholera vibriosis were not nationally notifiable until 2007 but have been reported in Florida since 1981. This may account for some of the differences between the epidemiology of vibriosis in Florida and elsewhere in the USA. Data were also not always complete on each individual. In Florida, case investigation and reporting is typically performed at the county-level, and there may be inconsistencies in the way data were collected. Moreover, patients or their families were not always able to recall details of food histories or other exposures. Almost 25% of reported cases had no known exposure, a huge gap in epidemiological data. Finally, we were unable to assess some factors related to vibriosis (e.g. hospitalization, treatment) since these variables were not included in our database.
The changing patterns of vibriosis noted in this and other studies may be related to increased awareness and surveillance, as well as higher water temperatures and other environmental factors. However, the increased incidence in vibrio infections highlights the need for continued and improved education and risk reduction for at-risk populations, with a new focus on wound-related infections. Because certain Vibrio spp. are associated with significant morbidity and mortality, especially in those with underlying health conditions, a continuing focus on increasing awareness and understanding of vibriosis in healthcare providers, high-risk populations with specific underlying health conditions, seafood consumers, and those with occupational or recreational exposures to seawater is needed.
ACKNOWLEDGEMENTS
The Council of State and Territorial Epidemiologists provided financial support for K. Weis during her Applied Epidemiology Fellowship.
DECLARATION OF INTEREST
None.











