Introduction
Since the early era of commercial logging, annual rates of deforestation in West Africa have been up to 70% above the global average (Martin Reference Martin1991), and Ghana is no exception. The former 82,000 km2 stretch of continuous forest in Ghana, which makes up the eastern part of the Upper Guinea forest, is now reduced to c. 15,000 km2 (Hawthorne and Abu Juam Reference Hawthorne and Abu Juam1993). Current satellite images (e.g. Google Earth) clearly show that the >200 state-protected high forest areas have become almost completely isolated islands in a mosaic of converted habitats and degraded forest remnants. The present ecological integrity of the Ghanaian forest biome is highly limited, with the largest contiguous area of inter-linked protected forests covering just 1,500 km2.
Current reforestation policies in Ghana focus on development of large-scale plantations, particularly Oil Palm Elaeis guineensis (national goal of 200 km2 per year), Cocoa Theobroma cacao and Rubber Hevea brasiliensis, and no protected area >5 km2 has been established in the high forest zone since the mid-1970s (Hawthorne and Abu Juam Reference Hawthorne and Abu Juam1993). This, combined with the past decades of accelerating subsistence farming throughout the forest zone of Ghana, makes establishment of new, nationally protected areas very unlikely. Furthermore, the fact that selective logging reaches even the most remote corners of any forest reserve highlights the importance of unreserved forest areas as extension zones that increase effective forest cover and improve linking networks between forest fragments. In effect, to maintain long-term forest ecosystem integrity in Ghana it is imperative to assess the conservation value of various land-use practices, and identify factors that facilitate the viability of forest bird populations and secure their genetic variability through local migration.
Unreserved tropical forest areas in Ghana and the rest of the world fall into six basic categories of land-use: 1) forest remnants; 2) various stages of farm/plantation abandonment and secondary successions, both subject to variable ‘non-timber forest-products’ (NTFP) harvesting; 3) annual crops; 4) perennial cash-crop plantations, either for subsistence or regularly harvested commercially; 5) perennial native or exotic tree plantations for long-term harvesting of timber or pulp, and 6) residential areas, including parks and gardens. Whereas land-uses 2 and 3 are usually products of the pan-tropical traditional shifting cultivation, various management practices characterise the production systems of perennial plantations, and reflect variations in their complexity and the diversity of the vertical vegetation structure. Hence, a principal task for combined bird conservation and sustained crop production is to identify regionally and locally adapted systems that address both interests (Gordon et al. Reference Gordon, Manson, Sundberg and Cruz-Angón2007). Since the 1960s, conservationists have steadily evaluated the ornithological importance of these land-uses (Elgood and Sibley 1964, Terborgh and Weske Reference Terborgh and Weske1969), though with a different emphasis in each part of the tropics. In a brief review of studies from the past two decades on impacts on the forest avifauna of land-uses (1–6) mentioned above, it is evident that isolated small forest remnants, maturing secondary forest (>5–10 years old), and abandoned plantations are superior forest bird habitats compared to early secondary successions (<5 years), non-arboreal or annual crops, and residential areas, that are only visited by few forest generalists (e.g. Blankespoor 1991, Kofron and Chapman 1995, Raman 2001, Marsden et al. Reference Marsden, Whiffin and Galetti2001, Hughes et al. Reference Hughes, Daily and Ehrlich2002, Jones et al. Reference Jones, Marsden and Linsley2003, Waltert et al. Reference Waltert, Bobo, Sainge, Fermon and Mühlenberg2005a, Bolwig et al. Reference Bolwig, Pomeroy and Mushabe2006, Marsden et al. Reference Marsden, Symes and Mack2006, Posa and Sodhi 2006, Soh et al. Reference Soh, Sodhi and Lim2006, Borges Reference Borges2007).
Several studies have unambiguously emphasised that industrially managed ‘sun’ plantations of cash crops such as e.g. Coffee Coffea spp., Cocoa, Oil Palm, and Rubber (Wilson and Johns 1982, Danielsen and Heegaard Reference Danielsen, Heegaard and Sandbukt1995, Aratrakorn et al. Reference Aratrakorn, Thunhikorn and Donald2006, Soh et al. Reference Soh, Sodhi and Lim2006), or timber monocultures such as Teak Tectona grandis or conifers (Carlson Reference Carlson1986, Beehler et al. 1987, Komar 2002, Sekercioglu 2002, Wijesinghe and Brooke 2005) do not benefit forest obligates. In contrast, recent data from both the New (Moguel and Toledo Reference Moguel and Toledo1999, Faria et al. Reference Faria, Laps, Baumgarten and Cetra2006, Komar Reference Komar2006) and Old World (Raman and Sukumar 2002, Raman Reference Raman2004, Waltert et al. Reference Waltert, Mardiastuti and Mühlenberg2004, 2005a,b) highlight the importance for forest bird conservation of traditionally managed ‘rustic’ cash-crops shaded by a planted, mono-dominant or variably-native intact canopy. Similarly, traditionally managed and floristically diverse agroforests (or ‘forest gardens’) of Rubber, native or exotic timber trees are also important for forest bird conservation in Indo-Malayan anthropogenic forest landscapes (Mitra and Sheldon Reference Mitra and Sheldon1993, Beukema et al. Reference Beukema, Danielsen, Vincent, Hardiwinoto and van Andel2007, Thiollay Reference Thiollay1995, Marsden et al. Reference Marsden, Symes and Mack2006), just as extensively managed, native mixed-timber plantations in Côte d'Ivoire (Waltert Reference Waltert2000) and Kenya (Farwig et al. Reference Farwig, Sajita and Böhning-Gaese2008) support many resident forest birds. As outlined in the previous sections, very few avifaunal studies on West African plantations exist, although plantation development is rapidly expanding here.
In this paper I assess the importance of shaded cash-crop (Cocoa and Coconut Cocos nucifera) and exotic timber (Gmelina arborea, Cedrela odorata) plantations for bird conservation, with particular reference to Guineo-Congolian understorey forest specialists in evergreen forests of south-west Ghana. Comparisons with similar studies from both the Old and New World tropics are made. Implications for off-reserve conservation management are discussed from global and regional perspectives. The present study was parallel to a study of the impact of selective logging and forest fragmentation on Ghanaian forest avifauna (Holbech Reference Holbech2005).
Study area and sites
The study was conducted from September 1993 to August 1995. Five plantation habitats were selected, adjacent to or within a protected area of south-west Ghana (Figure 1), covering wet evergreen (WE) and moist evergreen (ME) forest zones (Hall and Swaine Reference Hall and Swaine1976). WE-zone receives >2,000 mm of rain annually and the closed canopy reaches an average height of 35–40 m. Annual rainfall in ME-zone is 1,750–2,000 mm per year and the height of the discontinuous canopy ranges between 35 and 45 m. Both faunal and floral diversity is highest in the WE-zone, although the majority of large vertebrates occur in both zones. All selected sites lie in a lowland area with altitudes between 25 and 300 m a.s.l.
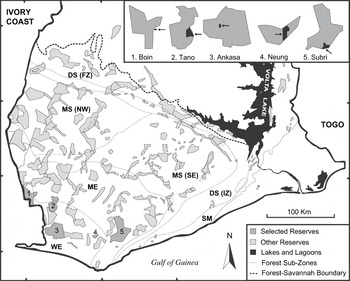
Figure 1. Map of the fragmented high forest zone in southern Ghana showing remaining reserves, forest type sub-zones and the selected study areas. Each study area is singly enlarged, indicating approximate size and location of plantations (black areas and arrows). Note that the scale of enlargements is not compatible between sites.
Two partially shaded cash crop plantations, ‘Boin Cocoa’ and ‘Ankasa Coconut’, as well as three exotic timber plantations, ‘Subri Gmelina’, ‘Neung Cedrela’ and ‘Tano Cedrela’, were included in this survey. Common to all five plantations was a well-developed secondary plant community made up of pioneer species (e.g. Musanga cecropioides, Cecropia peltata) or large forest trees. Undergrowth slashing had been abolished for several years giving these plantations a luxuriant appearance. The two Cedrela plantations formed the basis of a collapsed re-afforestation and agroforestry programme following illegal logging and farming. The Gmelina plantation was industrially managed but had some ‘biodiversity zones’ or ‘riparian strips’ conserved in marginal areas (steep slopes, narrow ridges and swamps), in which undergrowth slashing was limited. Table 1 outlines a general description of the 10 study sites. Detailed information on logging intensity for the five forest sites is found in Holbech (Reference Holbech2005).
Table 1. Description of the 10 study sites in the south-west Ghana.

a From Hall and Swaine (Reference Hall and Swaine1976): WE, wet evergreen (>2,000 mm); ME = moist evergreen (1,750–2,000 mm).
b Biogeographical isolation: The distance of transects to nearest other protected forest (0 km = joined with another forest).
c For plantations, the numbers refer to the crop height range, whereas those in brackets refer to maximum height of shading trees making up a discontinuous canopy.
d From Hawthorne and Abu Juam (Reference Hawthorne and Abu Juam1993): Genetic Heat Index (GHI) based on the rarity of tree species in Ghana.
Methods
Assessment of vegetation parameters in plantations
Three simple forest-bird habitat-quality parameters were assessed; 1) the density of native forest trees (planted or wild) >c. 0.5 m dbh; 2) density of planted exotics of any size, and 3) the extent of closed canopy. Living trees were counted systematically within 5 m of all transects. Hence, the number of counted trees per km transect, roughly equals tree density ha−1 (10 m × 1,000 m = 1 ha). The extent of closed canopy areas in each plantation was estimated by sectioning all transects for every 25 m and assessing whether each section passed through this habitat type. The percentage of closed canopy areas was then calculated as the cumulated closed-canopy sections divided by the total transect length for each plantation surveyed. Sections that passed though areas devoid of canopy were originally clear-cut for farming purposes but presently overgrown by dense vine tangles, ferns and in particular the invasive, perennial, exotic shrub Siam Weed Chromolaena odorata.
Mist-netting of birds
Mist netting at ground level (0–3 m) is repeatable and has no biases related to observer-skills and seasonal fluctuations in, for example vocalisation frequency, and is the best method for detecting cryptic, shy and silent species otherwise easily missed by audio-visual counts (Karr Reference Karr, Ralph and Scott1981, Derlindati and Caziani Reference Derlindati and Caziani2005). A limitation however, is that bird sampling is strongly biased towards lower storey birds and cannot be used to census whole forest bird communities (Poulsen Reference Poulsen1994, Remsen and Good Reference Remsen and Good1996). Our mist-nets were set 0–10 m from the centre-line of each transect, on both sides. Nets were 6, 9 or 12 m long, 4-shelved, with 15 mm mesh and 2.7 or 3.2 m high. The bottom of the lowest shelf was normally set 5–10 cm above the forest floor. Ten to 12 nets (90–126 m) were operated simultaneously. Nets were spaced at intervals of 100–150 m along each transect and kept open (closed during rains) from 06h00–06h30 to 17h00–18h30 and inspected regularly by a three-person team. To reduce problems associated with recaptures and net shyness, nets were moved every 2–4 days, according to the bird activity encountered. Birds were not banded but marked with a permanent ink colour pen on contour and down feathers, for recapture detection purposes.
Avifaunal classification-feeding guilds, forest habitat preferences and local status
All species were grouped according to food preference and foraging height (Brosset and Erard Reference Brosset and Erard1986, Gatter Reference Gatter1998), and foraging behaviour (from the literature, including ‘The Birds of Africa’, Vols. 1–7). A total of 11 major feeding guilds were identified (see Appendix in supplementary materials). Each species was also classified to the three forest-habitat preference categories defined in Bennun et al. (Reference Bennun, Dranzoa and Pomeroy1996), i.e. forest specialists (FF), forest generalists (F) and forest visitors (f). Additionally, the differentiation between primary (P), secondary (S) and riverine (R) forest birds based on Thiollay (Reference Thiollay, Diamond and Lovejoy1985) was also used. Largely, species-specific forest habitat categorisation follows Bennun et al. (Reference Bennun, Dranzoa and Pomeroy1996), with only seven exceptions. Birds of the same species may actually have slightly different habitat preferences within their range, particularly for birds with large ranges (Bennun et al. Reference Bennun, Dranzoa and Pomeroy1996); Dranzoa (Reference Dranzoa1998) categorised some 10 species differently to Bennun et al. (Reference Bennun, Dranzoa and Pomeroy1996) for Kenya/Uganda.
Three extensive bird surveys covering both evergreen and deciduous forest types in Ghana were used to determine the local status of birds (Beier et al. Reference Beier, Van Drielen and Kankam2002, Dowsett-Lemaire and Dowsett 2005a–j, Holbech Reference Holbech2005, including the present survey). Presence/absence data for each of the three surveys were assessed, and the percentage occurrence for each species was determined accordingly. Percentage occurrences in Beier et al. (Reference Beier, Van Drielen and Kankam2002) were based on n = 121 sampled transects, in Dowsett-Lemaire and Dowsett (2005a–j) on n = 10 reserves surveyed, and in Holbech (Reference Holbech2005) on n = 20 forest-zone habitats including the five plantations of the present study. The mean of the calculated three %-occurrences represents the general local status of a species in Ghanaian forests. Five local status categories were distinguished between; ‘rare’ with a mean occurrence ≥10.0%, ‘uncommon’ (10.1–25.0%), ‘fairly common’ (25.1–50.0%), ‘common’ (50.1–75.0%), and ‘widespread’ (≤75.1%). Rare and uncommon species were classified as of conservation importance, as these are area sensitive and restricted to the interior of the Ghanaian high forest.
Data analyses
As sampling efforts for each site were stratified according to its size and accessibility, the observed species richness could not be directly compared as species number and sampling efforts are highly correlated. Instead, three species richness estimators and one commonly used ecological diversity index were calculated using the computer software EstimateS version 8.0.0. (Colwell Reference Colwell2006). The estimators used follow recommendations by Colwell and Coddington (Reference Colwell and Coddington1994) on robustness towards sampling size, i.e. Chao 2 and Jack-knife 2, together with the Abundance-based Coverage Estimator (ACE). Additionally, the Shannon-Wiener index (H′) of diversity provides information on the evenness component of species diversity (Magurran Reference Magurran1988).
The richness-weighted α (log series) index of diversity was applied to assess bird guild diversity as this index has low sensitivity to sample size and a good ability to discriminate between sites (Magurran Reference Magurran1988). To assess species similarity between the ten sites, the quantitative Morisita-Horn index (CMH) was applied. This index is little biased by differences in sample size and species richness, and it is the most appropriate measure for large species assemblages with relatively few dominant and many rarely recorded species (Magurran Reference Magurran1988). A low similarity between two sites is equivalent to a high complementarity between these. As bird guild data on relative abundance and ecological diversity did not follow a normal distribution (skewness and kurtosis tests performed), these were compared using non-parametric Mann-Whitney U-tests (z distribution only for n > 20).
Results
Tree density and percentage of closed canopy
Whereas the closed canopy percentages in the two cash-crop plantations were fairly similar, the density of large trees in Boin Cocoa was almost twice that of Ankasa Coconut (Table 2 and Figure 2). The Tano Cedrela plantation had 50–100% more large forest trees than the two other plantations, whereas the density of exotics was lowest here. In contrast, the Subri Gmelina had the highest exotic-tree density, but also the lowest density of large forest trees. Hence, among the three exotic plantations, the density of exotics seems to be inversely proportional to large forest-tree density. The fact that Tano Cedrela had the highest density of large forest trees, yet a relatively low percentage of closed canopy areas, indicates that, as with cash-crop plantations, large forest trees were unevenly distributed here. In terms of overall large tree density (exotics and native), Neung Cedrela ranked as the exotic plantation offering lowest bird habitat quality, whereas Ankasa Coconut overall served as the poorest habitat for forest dependent birds. However, when considering the percentage of large forest trees Subri Gmelina represented the lowest forest bird habitat quality with only 29% forest trees and Tano Cedrela the richest (75%). Contrastingly, in terms of closed canopy percentage, Tano Cedrela was the most degraded exotic plantation, and Boin Cocoa the overall most degraded habitat. When combining the three determinants of habitat quality, Ankasa Coconut was the most degraded habitat, whereas Tano Cedrela was only slightly superior to the other two and fairly similar exotic plantations. Overall, Boin cocoa rated as an ‘intermediary’ bird habitat quality with fairly high forest tree density but also a relatively low proportion with closed canopy (<50%).
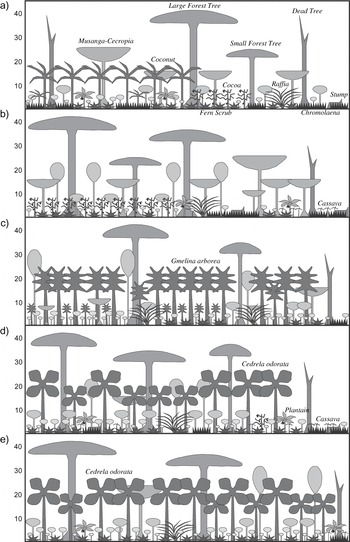
Figure 2. Schematic diagram of the structural vegetation profile of a) Ankasa Coconut, b) Boin Cocoa, c) Subri Gmelina, d) Tano Cedrela and e) Neung Cedrela. For simplicity, climbers and epiphytes are not shown. Strata height indicated in metres.
Table 2. Tree density (ha−1) and percentage of closed canopy areas in cash-crop and exotic tree plantations of south-west Ghana. Figures in brackets are percentage of all large trees (exotics and forest trees = 100%).

Overall relative bird abundance
Relative bird abundance is in this study defined as captures per net-metre-hours (NMH). Overall capture rates (Table 3) were significantly higher (U = 24.0, P < 0.05) in plantations (0.052 ± 0.016) compared to forest controls (0.0310 ± 0.004). Capture rates for the two cash crop plantations were 86–151% times that of their respective forest references, partly due to the Yellow-whiskered Greenbul Andropadus latirostris accounting for 34–67% of overall abundance. When excluding A. latirostris, only Ankasa Coconut remained 53% higher than Ankasa RR. Hence, understorey bird abundance was generally higher in Ankasa Coconut, whereas only one species dominated the captures in Boin Cocoa. Capture rates for Tano Cedrela and Subri Gmelina were c. 65% (39–56% exclusive of A. latirostris) higher than respective forest references, whereas Neung habitats did not differ significantly, indicating that understorey-bird abundance was relatively high in exotic plantations adjacent to large natural forests. In summary, overall bird abundance appeared highest in cash-crop plantations, second highest in exotic plantations and lowest in reserves, though dominated by a few species in plantations, particularly A. latirostris, Green Hylia Hylia prasina, Olive Sunbird Cyanomitra olivaceus and Red-bellied Paradise Flycatcher Terpsiphone rufiventer.
Table 3. Diversity statistics (using EstimateS with 100 randomisations without replacement; Colwell Reference Colwell2006) for the five plantation habitats and their respective forest references in south-west Ghana. In an overall assessment of habitat ranking, a score index has been calculated for each of the four diversity measures (points of 1–10), and the cumulated score expresses the overall ecological diversity of each habitat. Figures in brackets indicate the relative (%) overall diversity score of each plantation compared to forest associate.

1 Relative abundance = birds netted per net-meter-hours.
2 Abundance-based Coverage Estimator of species richness.
3 Chao 2 estimator of species richness.
4 Jack-knife 2 estimator of species richness.
5 Shannon-Wiener index of species diversity (H′).
Species richness and ecological diversity
Among the four diversity descriptors, only Jack 2 was significantly higher (U = 25.0, P < 0.05) in forests compared to respective plantations, whereas ACE and Shannon showed a ‘probably significant’ (U = 23.0, P = 0.05) difference between forests and plantations (Table 3). When combining the four diversity descriptors, all reserves were superior to their respective plantation. Among the five plantations, Tano Cedrela ranked highest for a combined score of all four descriptors, making up 96% of its forest reference, followed by Ankasa RR with 61% of its associated forest. In contrast, Neung Cedrela displayed by far lowest diversity scores, making up <25% of that of the Neung FR, whereas Boin Cocoa and Subri Gmelina were almost equally diverse. Hence, plantations situated outside reserves (Boin Cocoa) or adjacent to small impoverished reserves (Neung Cedrela) represented the poorest forest avifaunas.
Species similarity and complementarity
Species similarity was highest between Neung FR and Subri Gmelina (CMH = 0.981), and lowest between Boin Cocoa and Tano FR (CMH = 0.457) (Table 4). Overall, similarity was highest among forests (0.902 ± 0.052), and lowest on average between forests and plantations (0.791 ± 0.143), yet the average similarity between forests and respective plantations was relatively high (0.811 ± 0.155), suggesting that plantations were colonised by surrounding forests (Table 5). The average similarity among exotic plantations (0.890 ± 0.051) was high, indicating that such habitats supported similar bird fauna irrespective of the nearby forest. Exotic plantations and their respective forest associations were also similar (0.902 ± 0.054), whereas cash-crop plantations were more complementary to nearby forest habitats (0.675 ± 0.170). When comparing mean similarity figures in Table 5, only one comparison was significantly different (z = 2.48, P = 0.01, n = 35), namely between all forests and all forests versus plantations These data suggest that complementarity among plantations was higher than among forests in general, just as cash crops were more complementary to respective forests than were exotics.
Table 4. Species similarity between five plantation habitats and their respective forest associates in south-west Ghana, as shown by the quantitative Morisita-Horn index (CMH).

CMH = 2Σ (an i · bn i)/(da + db) · aN · bN, where da = Σ an i2/aN 2 and db = Σ bn i2/bN 2
Table 5. Mean and standard deviation (SD) of similarity values between different habitat categories: forests, exotic (Gmelina or Cedrela) and cash crop (Cocoa or Coconut) plantations, based on values obtained in Table 4.

Distribution of feeding guilds
In terms of relative abundance (captures NMH−1) only lower strata (<10 m) foliage-gleaners were significantly more abundant in plantations (U = 24.0, P < 0.05) (Table 6). Lower strata birds were generally also significantly more abundant in plantations (U = 24.0, P < 0.05), due to the high dominance of A. latirostris, an understorey (0–3 m) foliage-gleaning omnivore. However, when this species is removed from the data, the difference between plantations and forests was insignificant. With respect to within-guild diversity (α-log series index) only mid-level (10–30 m) foliage-gleaners were probably more diverse in plantations (U = 23.0, P = 0.05). These results indicate that trophic organisation of the understorey bird community in forests and plantations were similar, and emphasise the potential of these plantations for some feeding guilds that are vulnerable to forest disturbance, e.g. salliers, bark- and litter-gleaners.
Table 6. Mean (± SD) relative abundance (captures per 1,000 NMH) and species diversity (α log-series) for 23 bird guilds found in five forest and five plantation habitats of south-west Ghana. Significant differences between forests and respective plantations were determined by Mann-Whitney U-tests (2-tailed, n = 5). Significant results in bold. ID = insufficient data; ns = not significant (P > 0.05).

Car = Carnivore, Fru = Frugivore, Ins = Insectivore, Nec = Nectarivore, Gra = Granivore, Pis = Piscivore, Omn = Omnivore, GU = Ground-understorey (0–3 m), LM = Lower mid-storey (3–10 m), UM = Upper mid-storey (10–30 m), C = Canopy (>30 m).
Distribution of birds and forest habitat preferences
Of 78 species netted, 47 (60%) were forest specialists (FF), 30 (39%) forest generalists (F) and only one (1%) a forest visitor (f). Relative abundance (captures NMH−1) of FF-birds was not significantly different between forests and plantations, whereas F-birds were significantly (U = 25.0, P < 0.05) more abundant in plantations (Table 6). In terms of diversity, no differences between forests and plantations were established. Only one f-species was netted, Grey-backed Camaroptera Camaroptera brachyura (Subri FR), and no non-forest species were netted anywhere. Hence, plantations supported rather diverse assemblages of FF-species, although F-species in terms of relative abundance dominated here. The F-dominance was particularly attributed to A. latirostris but also Little Greenbul Andropadus virens and Olive Sunbirds.
Distribution of ant-followers
Birds following army ants are often sensitive to forest disturbance (Roberts et al. Reference Roberts, Cooper and Petit2000, Barlow et al. Reference Barlow, Peres, Henriques, Stouffer and Wunderle2006). Among 16 ant-following species netted, all plantations had fewer of these birds compared to forest associates (mean 69%), ranging from 36% (Neung Cedrela) to 91% (Subri Gmelina), although the comparative tests on relative abundance and diversity were both insignificant (Table 6). These data indicate that army ants occur in plantations at relatively high densities with the exception of the small and heavily disturbed Neung Cedrela plantation.
Distribution of red list endemics and other species of conservation importance
Three species categorised by IUCN (2001) as threatened Upper Guinea Forest endemics were netted in the present study: Yellow-bearded Greenbul Criniger olivaceus, Green-tailed Bristlebill Bleda eximius and Rufous-winged Illadopsis Illadopsis rufescens, all specialised understorey insectivores (ant-follower or bark-gleaner). The mean abundance of these birds was significantly higher for all forest sites compared to plantation associates (U = 25.0, P < 0.05) (Table 6). Only four individuals were netted in three plantations. Hence, plantations are only marginal habitats for these birds, and it is most likely that their presence here relied on the proximity of surrounding high forest.
Of the 78 species netted, four species were classified as ‘rare’ and 15 as ‘uncommon’. Cassin's Flycatcher Muscicapa cassini was also determined as ‘uncommon’, but is a riverine species and hence excluded from the analysis of species of conservation importance. Two red list endemics were determined as ‘fairly common’, but were also included as important for conservation in Ghana due to their critical conservation status. Hence, the total number of species of conservation importance was 20, and high numbers of these birds are indicative of a diverse and complex forest bird community. The majority of these 20 species are FF-species (80%) and Guineo-Congolian or Upper Guinea endemics (75%).
The mean abundance of species of conservation importance was probably significantly higher (U = 23.0, P = 0.05) in forests than plantations (Table 6). Sixteen species of conservation importance were netted in forests (12 exclusively here), and eight in plantations, i.e. 50% of the number found in forests. The mean percentage of these species found in plantations compared to forest associates was 26% (range 17–43%). Despite relatively low densities, the presence of these birds in plantations is indicative of a fairly complex bird community. The African Piculet Sasia africana and Lemon-bellied Crombec Sylvietta denti (both FF/P-species) were netted in only three plantations, but not in 15 Ghanaian forest reserves (Holbech Reference Holbech2005).
Discussion
Factors influencing the conservation importance of plantations for forest birds
According to general theories of biogeography and species diversity (e.g. MacArthur Reference MacArthur1972, Diamond Reference Diamond1973), four prime factors may determine resident forest bird diversity in plantations; 1) distance to the nearest source of forest birds; 2) the bird community at that source; 3) habitat quality of corridors (Sieving et al. Reference Sieving, Willson and de Santo2000, Jansen 2005, and 4) habitat quality of the plantation, which is often highly linked to size of the area. Factors 1–3 are all determinants of bird dispersal magnitude into plantations, whereas factor 4 reflects the capability of plantations per se to sustain viable forest bird populations. Where plantations are close to and/or well connected to little-disturbed natural forests, it is difficult to determine whether birds only make temporary and spatially facultative use of the plantations, or are actually persisting there. Resolving that issue would require monitoring individual movements or identifying breeding territories and feeding home ranges. Hence detection of the presence of birds does not necessarily indicate bird persistence or functional habitat use (Peh et al. Reference Peh, de Jong, Sodhi, Lim and Yap2005), particularly in the case of highly mobile species with large home ranges, e.g. raptors (Thiollay Reference Thiollay1993), pigeons, hornbills and parrots (Kofron and Chapman 1995, Marsden and Pilgrim Reference Marsden and Pilgrim2003). However, when plantations have been isolated for decades, in areas with no significant nearby forest, the presence of forest birds likely reflects their adaptability to the plantation habitat (Greenberg et al. Reference Greenberg, Bichier, Cruz-Angón and Reitsma1997a,b, 2000). This is particularly true for species with low mobility and high territoriality in small areas, e.g. for many edge-shy, terrestrial or understorey insectivores.
All but one of the five plantations were situated within moderately logged natural forest, and as these were relatively more diverse and similar compared to the more isolated Boin Cocoa, it suggests use rather than forest bird persistence in these plantations. Several recent studies around the world have indicated that distance to nearby forest is the major contributing factor to forest bird diversity in plantations (Naidoo Reference Naidoo2004, Sodhi et al. Reference Sodhi, Koh, Prawiradilaga, Darjono, Tinulele, Putra and Tan2005a, Tejeda-Cruz and Sutherland Reference Tejeda-Cruz and Sutherland2004, Waltert et al. Reference Waltert, Bobo, Sainge, Fermon and Mühlenberg2005a, Soh et al. Reference Soh, Sodhi and Lim2006, Bolwig et al. Reference Bolwig, Pomeroy and Mushabe2006). This apparent distance-effect depends on the extensiveness or patchiness of nearby forests, as indicated by plantation studies adjacent to small patches of remnants (Reitsma et al. Reference Reitsma, Parrish and McLarney2001, Waltert et al. Reference Waltert, Mardiastuti and Mühlenberg2005b, Lindenmayer et al. Reference Lindenmayer, Fisher and Cunningham2005). In line with this, sensitivity to distance isolation in matrix ecotones depends on the mobility and territoriality of each species as mentioned above, whereby aggregates of bird community species diversity may not always be simply correlated with forest coverage alone (Lindenmayer et al. Reference Lindenmayer, Fisher and Cunningham2005). Finally, distances to nearest natural forest play minor roles in industrial monoculture plantations (no canopy shade and heavy undergrowth weeding) situated in heavily degraded areas with very poor forest habitat connectivity (Aratrakorn et al. Reference Aratrakorn, Thunhikorn and Donald2006).
In the present study, measures of similarity strongly indicate that exotic plantation avifaunas were closely related to their adjacent forest compared to other distant forests and plantations. In particular the depauperate avifauna of Neung Cedrela clearly reflected the already highly impoverished degraded bird habitat of adjacent Neung FR. Peh et al. (Reference Peh, de Jong, Sodhi, Lim and Yap2005) also found higher species diversity in anthropogenic matrix landscapes close to 800 km2 large forest compared to 300 km2 forest, just as Faria et al. (Reference Faria, Laps, Baumgarten and Cetra2006) found higher forest bird diversity in plantations situated in an extensively forested zone compared to similar plantations in areas with only 4.8% remnants of 1–300 ha. At the microscale level, the size of forest patch remnants has also been shown to affect overall bird diversity in plantations relatively more than the mere distance from remnants (Tubelis et al. Reference Tubelis, Lindenmayer and Cowling2004).
Although the relative species richness of the Boin Cocoa ranked lowest among the five plantations, a number of FF-species were present up to >500 m from the forest edge, e.g. White-crested Hornbill Tropicranus albocristatus, Red-headed Dwarf-kingfisher Ceyx lecontei, Red-tailed Greenbul Criniger calurus, Western Bearded Greenbul Criniger barbatus, White-tailed Ant-thrush Neocossyphus poensis, Brown-chested Alethe Alethe poliocephala, and Blue-headed Crested-flycatcher Trochocercus nitens. Many of these birds were associated with army-ant swarms. The presence of these species suggests some level of habitat connectivity between the very diverse Boin FR and the shaded cocoa plantation by virtue of the complex matrix of forest remnants, secondary forest and farmland. Habitat connectivity has been shown to play a vital role for dispersal of forest specialists between forest remnants and various types of plantation agro-ecosystems in otherwise heavily disturbed matrix landscapes (Estrada et al. Reference Estrada, Coates-Estrada and Meritt1997, Graham Reference Graham2001, Raman Reference Raman2004, Castelletta et al. Reference Castelletta, Thiollay and Sodhi2005, Faria et al. Reference Faria, Laps, Baumgarten and Cetra2006), and may in some cases moderate the pronounced decline of forest birds related to distance isolation of small and otherwise ecologically disintegrated habitat units (Reitsma et al. Reference Reitsma, Parrish and McLarney2001). Due to their close proximity and excellent connectivity, it is likely that the Boin Cocoa and surrounding land-use matrix may increase the functional habitat for FF-species deriving from the nearby Boin FR, but long-term capture-mark-recapture is necessary to evaluate persistence of these species in the plantation.
Tree plantations and forest bird conservation – a pan-tropical comparative evaluation
It has recently been shown that the structural and floristic diversity of plantations (factor 4) is positively correlated with forest bird species diversity (Raman and Sukumar 2002, Carlo et al. Reference Carlo, Collazo and Groom2004, Waltert et al. Reference Waltert, Bobo, Sainge, Fermon and Mühlenberg2005a, Cruz-Angón and Greenberg Reference Cruz-Angón and Greenberg2005). A number of inter-related factors may contribute to the vertical and horizontal vegetation complexity of plantations, including choice and density of crop species, plantation age, and most importantly, the intensity of management and control of the secondary plant community. Basically two major structural types of plantations can be identified in the tropics; 1) where the primary crop makes up the understorey level (<c. 5 m) of the vegetation, and is either non-shaded or shaded by a canopy of native or planted trees (e.g. Coffee, Cocoa); or 2) where the primary crop variably makes up the canopy level (>c. 10 m), and in which native plants may variably dominate the understorey together with seedlings and saplings of the crop (e.g. Rubber, Coconut, Oil Palm, Teak, Gmelina). Hence, cash-crop plantations may conform to both ‘understorey-tree’ plantations (e.g. Coffee) and ‘canopy-tree’ plantations (e.g. Oil Palm). On the other hand Rubber or timber plantations are always the latter. I have found no published bird studies in combined timber and cash-crop plantations, perhaps because the two are incompatible in terms of problems with nutrient competition or damage to cash crops during timber harvest.
According to the above division I have compiled data on biogeography, vegetation structure and forest bird conservation importance from 23 understorey-tree and 23 canopy-tree plantations throughout the tropics, including the five Ghanaian plantations (Tables 7 and 8). Though this is a rather problematic task, the plantations are ranked according to their relative importance in providing suitable habitats for forest birds, irrespective of the degree of forest bird persistence (use of plantations indicated by their presence). The ranking has particularly emphasised the extent (%) to which true forest birds and/or species of conservation importance are found in plantations compared to native primary or variably disturbed forest. Other complementary conservation importance indicators are species similarity (S = various kinds) and diversity (H′). The main bias in this comparison comes from whether plantations are compared to large tracts of primary forest or heavily disturbed forest fragments, in the latter case explaining why plantations can attain very high percentages of important forest species compared to already depleted ‘control’ sites. However, even though some of the compared parameters vary between studies, this analysis provides a useful general outline of patterns, and is at least able to discriminate between plantations with very high versus very low importance.
Since forest bird diversity is low in any non-shaded (sun) understorey-tree plantation, the crop per se plays a minor role for forest bird conservation (Table 7). However, in shaded understorey-tree plantations, it may be that Cardamom Elettaria cardamomum and Coffee are more compatible with forest bird conservation than Cocoa. If true, the superiority of Cardamom and Coffee may be related to a higher degree of co-existence of these crops with a more diverse sub-canopy plant community, as these crops are, on the average, lower and less shady than Cocoa trees. In the rustic Ghanaian Cocoa plantation, understorey-weeding had been abolished for several years allowing dense under- and mid-storey regrowth in open canopy patches (~55%). In otherwise intensively managed rustic plantations elsewhere in Ghana, the 5–10 m high Cocoa trees inhibit most plant growth, creating sparse undergrowth dominated by ferns and herbs. The understorey avifauna of such plantations is depauperate and dominated by a few abundant forest generalists.
Table 7. Comparison of 23 understorey tree-plantations in the Old and New World tropics, including the rustic cocoa plantation of this study in Ghana (*). The plantations are ranked in order of relative forest bird conservation importance: H = high (>75% or >50% of respectively all species (AS) or (priority) forest species (FS) present in variably disturbed native forest, similarity index (S) > 0.50, diversity index (H′) > 2.50); M = moderate (AS = 50–75%, FS = 25–50%, S = 0.25–0.50); L = low (AS < 50%, F < 25%). Understorey and canopy codex: D = Diverse, multi-storied, large climbers, epiphytes; MD = moderate diverse, semi-storied, shrubs, vines; P = poor, single storey dominated by plantings, herbs and/or grasses.

1 Some forest tree emergents up to 40–50 m (>100 years old); good habitat connectivity to nearby forest.
2 Greenberg et al. (Reference Greenberg, Bichier, Cruz-Angón and Reitsma1997a): Mono-shaded coffee (40–50%) by mainly Inga or Gliricidia spp., and respectively 45 and 29 other tree species. Sun coffee negligible canopy cover. Rustic cardamom shaded (69%) by native secondary forest; all systems in a matrix of forest remnants and secondary shrubbery (matorral).
3 Raman (Reference Raman2004): Cardamom with native canopy (88% cover) and coffee under mixed exotic/native canopy (69% cover), both within a matrix of forest remnants and plantations, and adjacent to 20–26 km2 large forest blocks and/or small remnants (0.3–650 ha).
4 Beehler et al. (1987): Incomplete canopy of remnant forest trees and an exotic tree in a disturbed forest area.
5 Faria et al. (Reference Faria, Laps, Baumgarten and Cetra2006): Rustic ‘cabrucas’ situated in an area with 4.8% small (1–300 ha) forest remnants (Illneus) or in an area with >50% large (up to 110 km2) remnants (Una).
6 Raman and Sukumar (2002): Shaded old plantation abandoned for 5–15 yrs; canopy cover 72–91% of forest trees and exotics.
7 Waltert et al. (Reference Waltert, Bobo, Sainge, Fermon and Mühlenberg2005a): Shaded coffee or cocoa plantations with forest tree density of c. 25 h−1 (dbh > 0.50m).
8 Greenberg et al. (Reference Greenberg, Bichier and Sterling1997b): Rustic coffee under native almost intact canopy. Mono-shaded coffee dominated by Inga spp.; very few nearby closed forest remnants along ridges.
9 Waltert et al. (Reference Waltert, Mardiastuti and Mühlenberg2004): Mono-shaded cocoa by mainly Gliricidia spp., adjacent to closed forest.
10 Tejeda-Cruz and Sutherland (Reference Tejeda-Cruz and Sutherland2004): Mono-shaded (41%) coffee dominated by Inga spp. Rustic coffee shaded (60%) by an almost original canopy cover. Sun coffee no shade at all. All plantations situated in a buffer zone with 60% dense forest.
11 Estrada et al. (Reference Estrada, Coates-Estrada and Meritt1997): Native shaded plantations in a matrix area with isolated forest remnants (1–2,000 ha) and good habitat connectivity through live fences.
12 Reitsma et al. (Reference Reitsma, Parrish and McLarney2001): Mono- or multi-species shaded; matrix of forest patches (1–200 ha), farms and pastures.
13 Greenberg et al. (Reference Greenberg, Bichier and Cruz-Angón2000): Plantations with 60 species of planted shade trees (no single dominant species); no nearby closed forest, not even remnants.
14 Bolwig et al. (Reference Bolwig, Pomeroy and Mushabe2006): Commercial large-scale plantations in a matrix landscape, with forest remnants > 1 km2.
15 Relatively high diversity of woodland and migrant forest species, but few or no resident forest specialists.
Table 7 compares three basic shade types for understorey-tree plantations; 1) mono-type shading by a single shade tree (e.g. Inga or Gliricidia); 2) shade provided by a semi-rustic canopy consisting of up to 60 planted tree species, and 3) rustic canopy cover of almost intact native forest. When comparing plantations across these categories, taking account of biogeographical factors, data indicate that rustic plantations are superior bird habitats compared to the two other agro-forestry systems that are similar in terms of forest bird conservation value. Hence, planting one dominant or several shade species in a mixture appears to play a minor role compared to leaving the natural canopy as shade.
Among industrial canopy-tree plantations, Oil Palm seems to provide a less suitable forest bird habitat than Rubber (Table 8), probably due to a thin-tall Rubber tree canopy that transmits enough sunlight for diverse sub-canopy plant growth. Industrial Oil Palm and Cocoa plantations form a very dense evergreen canopy that restrains a dark and poorly developed shrub layer (Peh et al. Reference Peh, Sodhi, de Jong, Sekercioglu, Yap and Lim2006). Although Aratrakorn et al. (Reference Aratrakorn, Thunhikorn and Donald2006) describe Oil Palm and Rubber as having similar bird communities, sallying and litter-gleaning insectivores were more abundant in Rubber. Similarly, Peh et al. (Reference Peh, Sodhi, de Jong, Sekercioglu, Yap and Lim2006) observed bark-gleaners in Rubber but never in Oil Palm. In Ghana, Coconuts did not form a closed canopy, and as Coconut crowns transmit more light than Oil Palm, a luxuriant understorey flourished below the tall palms. Coconut palms easily attained heights of 15–20 m and co-existed with a diverse undergrowth of >10 m including several forest interior birds, e.g. Forest Robin Stiphrornis erythrothorax, Finsch's Flycatcher-thrush Stizorhina finschi, Bleda eximius and Blue-headed Dove Turtur brehmeri. Marsden and Pilgrim (Reference Marsden and Pilgrim2003) found diverse hornbill and parrot assemblages in similar coconut agro-forests of PNG, in which four parrot species fed on the coconut flowers.
Table 8. Comparison of 23 canopy tree-plantation systems in Southeast Asia and Africa, including the four plantations of the present study in Ghana (*). The plantations are ranked in order of relative forest bird conservation importance: H = high (> 75% or > 50% of respectively all species [AS] or [priority] forest species [FS] present in variably disturbed native forest, similarity index [S] > 0.50, diversity index [H′] > 3.50); M = moderate (AS = 50–75%, FS = 25–50%, S = 0.25–0.50, H′ = 2.50–3.50); L = low (AS < 50%, FS < 25%, H′ < 2.50). Understorey and canopy codex: D = Diverse, multi-storied, large climbers, epiphytes; MD = moderate diverse, semi-storied, shrubs, vines; P = poor, single storey dominated by plantings, herbs and/or grass.

1 Some forest tree emergents up to 40–50m (>100 years old).
2 Waltert (Reference Waltert2000): Terminalia/Triplochiton spp. (native timber trees) in abandoned cocoa and deforested areas within a forest reserve.
3 Beukema et al. (Reference Beukema, Danielsen, Vincent, Hardiwinoto and van Andel2007): Traditionally rubber-enriched secondary fallow, 25 years after slash-and-burn agriculture; 36% of canopy rubber trees (dbh > 0.1m), of which 66% were still being tapped.
4 Mitra and Sheldon (Reference Mitra and Sheldon1993): Thin canopy of only Albizia falcataria (exotic), a with rich, woody and tangled undergrowth, situated 1–2 km from circumscribed stands of primary forest.
5 Thiollay (Reference Thiollay1995): Traditionally managed multi-species ‘garden forests’ that originated 150 years ago. Rubber (exotic): 30–80% Hevea; Durian (exotic): <70% canopy cover of mainly Durio zibethinus; Damar (native): Continuous canopy of 56–80% Shorea javanica (Dipterocarp). Plantations host at most 20–40 tree species ha−1.
6 Beehler et al. (1987): Sub-mature plantation with uniform canopy and thin mid-storey in a disturbed forest area.
7 Komar (2002): 3 km2 Mexican cypress situated in between montane cloud forest (25 km2) and sub-montane pine-oak forest (150 km2).
8 Carlson (Reference Carlson1986): Pinus radiata (exotic) monocultures in a matrix of cultivated fields and primary forest remnants.
9 Peh et al. (Reference Peh, Sodhi, de Jong, Sekercioglu, Yap and Lim2006): Large-scale mono-culture plantations on 30–35 years old forest clear-cut land, but adjacent to extensive contiguous forest areas, and interspersed with subsistence farming (open areas).
10 Sodhi et al. (Reference Sodhi, Malcolm, Prawiradilaga, Darjono and Brook2005b): 120 ha Pinus merkusii native plantation in heavily degraded Java (2.3% forest left), close to 255 km2 large remnant.
11 Danielsen and Heegaard (Reference Danielsen, Heegaard and Sandbukt1995): Forest completely converted to industrial plantations with sparse undergrowth due to regular weeding.
12 Aratrakorn et al. (Reference Aratrakorn, Thunhikorn and Donald2006): Forests completely converted to industrial plantation with sparse undergrowth.
13 Bell (Reference Bell1979): Thin continuous canopy of only teak (exotic) with few epiphytes, lichens and vines. Understorey of planted Laucaena legume.
14 Relatively high diversity of Palaearctic migrant forest species, but few or no resident forest specialists.
Comparing similarly managed coniferous and deciduous wood plantations suggests that mature conifers represent inferior forest bird habitats (Table 8), reflecting the depauperate understorey beneath the dense evergreen canopy (Carlson Reference Carlson1986, Komar 2002, Sodhi et al. Reference Sodhi, Malcolm, Prawiradilaga, Darjono and Brook2005b). It appears that the choice of deciduous species is less important than the age, and hence height, of the trees (Bell Reference Bell1979, Mitra and Sheldon Reference Mitra and Sheldon1993). The choice of a native versus exotic species in mono- and poly-cultures (<5 spp.) seems also to be of limited importance for forest birds, so far as plantings co-exist with a structurally and floristically diverse secondary plant community, particularly at the sub-canopy levels. In commercial oil palm and rubber plantations of Thailand, the presence of even sparse undergrowth has a significant positive impact on the forest avifauna (Aratrakorn et al. Reference Aratrakorn, Thunhikorn and Donald2006).
Land-use management implications and future research focus
Substantial evidence from the tropics, including Ghana, emphasises that although complex plantations may serve as important avifaunal supplements or complements, they can never be a substitute for natural forest. In effect, this implies that buffer zones or corridors of appropriately managed agro-silvicultural ecosystems, identified earlier in this paper, may significantly increase the functional habitats and hence effective conservation area for many restricted range forest specialists, otherwise confined to small or heavily disturbed forests.
In the case of Ghana, I believe that such diverse agro-ecosystems may increase impoverished populations of forest specialists above the minimum viable threshold, and actually link up nearby (<5 km apart) otherwise isolated reserves currently surrounded by intensive sun Cocoa plantations and indiscriminate slash-and-burn shifting cultivation. Future research should therefore focus on finding the optimum state of agro-silvicultural management in relation to avifaunal conservation, through cost-benefit analyses that integrate costs of weeding, pesticides and fertilizers (Fitzherbert et al. Reference Fitzherbert, Struebig, Morel, Danielsen, Brühl, Donald and Phalan2008). In particular, more information is needed on how much a secondary plant community affects crop yields and whether the optimum level of plant control can be maximized in terms of crop varieties, crop mixtures, bio-fertilizers and biological pest control. In terms of fertilizers, many rainforest trees and legumes are able to enrich leached tropical soils through mycorrhizal-mutualistic symbiosis, emphasising the need for more trees on the farm for other reasons than bird conservation.
Acknowledgements
This study was financed by a Ph.D. grant provided by Danida (No. 104.Dan.8/606), in collaboration with University of Copenhagen, Denmark and University of Ghana (UGL). My utmost gratitude goes to Ghana Wildlife Division (GWD) and Ghana Forestry Commission for granting research permits, and special appreciation for invaluable field assistance from the late Mr. Joseph Amponsah (GWD), and supervision by Prof. Y. Ntiamoa-Baidu, Department of Zoology (UGL). Dr. Phil. Jon Fjeldså, Zoological Museum, Copenhagen, Dr. Mike Dyer, and the BCI editor kindly provided constructive criticism and editing of earlier manuscripts.














