INTRODUCTION
The rate of tuberculosis (TB) in Spain is among the highest of industrialized countries [1–Reference Caminero3]. It is well known that drug addicts have a higher risk of TB and that alcoholism and intravenous drug use are among the main factors associated with TB [Reference Friedman4–Reference Moreno6]. Moreover, intravenous drug use has been the main category of human immunodeficiency virus (HIV) transmission in Spain [7], and the elevated incidence of TB in patients infected with HIV is also known, which increases the risk of developing tuberculosis up to 100-fold [Reference Díez2, Reference Muga8, Reference Selwyn9].
The diagnosis and treatment of latent infection is very important in the control of TB. For more than 100 years the tuberculin skin test (TST) or Mantoux test has been the only method used in its detection [Reference Horsburgh10]. The TST uses a protein purified derivate (PPD) that is a mixture of antigens, many of which are shared with Mycobacterium tuberculosis, M. bovis, Bacille Calmette-Guérin (BCG), and other non-tuberculosis mycobacteria; the limitations of TST are well known: low specificity in individuals vaccinated with BCG or in those exposed to other mycobacteria; sensitivity may be lessened in immunocompromised patients. On the other hand a booster effect may be produced by repeated use of TST and errors in the reading and interpretation of the results may occur. A further inconvenience is the need for a second visit for reading the results [Reference Duncan11, Reference Richeldi12].
Immunodiagnostic methods for detecting TB infection are based on the in vitro quantification of the cellular immune response in response to specific M. tuberculosis antigens [Reference Converse13, Reference Pai, Riley and Colford14].
In the first generation of in vitro tests the same antigen was used as in the TST, which caused some limitations [Reference Converse13]. The new versions use antigens more specific for M. tuberculosis such as early secretory antigen target-6 (ESAT-6), culture filtrate protein-10 (CFP-10) and TB7.7 antigen; the genes which encode ESAT-6 and CFP-10 are found in a segment named the region of difference 1 (RD1) of the M. tuberculosis genome and TB7.7 is encoded in RD11 which are not present in the genome of M. bovis, BCG or other non-tuberculosis mycobacteria such as M. avium [Reference Pai, Riley and Colford14]. Other mycobacteria that have been shown to induce T-cell responses to ESAT-6 and CFP-10 are M. marinum and M. kansasii [Reference Arend15].
There are two tests marketed for the in vitro diagnosis of TB based on M. tuberculosis-specific antigens: QuantiFERON TB Gold In-Tube (Cellestis Ltd, Australia) which uses enzyme immunoassay (EIA) techniques to measure the production of interferon-γ by the T cells in whole blood in response to ESAT-6, CFP-10 and TB7.7 and the T-SPOT.TB test (Oxford Immunotec Ltd, UK) that uses the enzyme-linked immunospot (ELISPOT) technique to determine the T cells that produce interferon-γ in response to the M. tuberculosis-specific antigens ESAT-6 and CFP-10 [Reference Richeldi12].
The usefulness of the tests in identifying latent infection by M. tuberculosis has been established [Reference Menzies, Pai and Comstock16, Reference Lalvani17]; however, there exists little information in some populations such as immunocompromised patients and drug users.
The objective of this study is to analyse TB infection rates in patients at high risk of infection using two interferon-γ techniques based on TB-specific antigens.
MATERIAL AND METHODS
Study population
Patients admitted to a detoxification unit in a tertiary hospital between February 2006 and May 2007 were included in the study. The patients came from different outpatient centres for the treatment of alcohol and drug abuse in metropolitan Barcelona. All subjects gave their consent for participation in the study.
Methods
Sociodemographic characteristics, as well as information on the history of alcohol and drug abuse were collected from all patients. For medical history patients were asked about previous TB disease or TB infection. Upon admission blood samples were taken for HIV serology (EIA and Western blot), RNA-HIV, CD4 lymphocytes and interferon-γ tests.
TST was carried out by means of intradermal administration of 2 U of PPD-RT23 in the forearm, except where the subject had a history of culture-proven previous TB disease or previous positive TST; the reading of TST was performed 48 h later and was considered positive if the induration was ⩾5 mm in HIV-positive subjects and ⩾10 mm in HIV-negative subjects. During admission active TB was ruled out in all cases by means of a chest X-ray and three sputum samples which were analysed using the Ziehl–Neelsen stain and Lowenstein–Jensen culture. If subjects presented with fever and/or constitutional symptoms, extrapulmonary localization of TB was also excluded.
The participants were assessed for TB infection with two interferon-γ techniques based on M. tuberculosis-specific antigens (EIA); QuantiFERON-TB Gold In-Tube (Cellestis Ltd) and ELISPOT (T-SPOT.TB) (Oxford Immunotec Ltd).
QuantiFERON-TB Gold In-Tube
Three millilitres of blood were distributed in three test tubes with anticoagulant, one of which contained ESAT-6-, CFP-10- and TB7.7-specific antigens, another with saline solution (negative control) and the third with phytohaemagglutinin (positive control). These were left to incubate at 37°C overnight. After incubation the plasma was separated by centrifugation and kept frozen (−20°C) until required.
The production of interferon-γ, expressed in IU/ml, was determined by ELISA (enzyme-linked immunosorbent assay) and analysis software from QuantiFERON was used to obtain the results. The value obtained was deduced in the negative control from the values obtained in the test tubes stimulated with mitogen and with the specific TB antigens. Values >0·35 IU/ml were considered positive in the sample stimulated with TB antigens. The result of the test was considered indeterminate if the production of interferon-γ after stimulation with phytohaemagglutinin was <0·5 IU/ml and the sample stimulated with TB antigen was <0·35 IU/ml.
T-SPOT.TB
The mononuclear cells were separated by density gradient centrifugation from a sample of 8 ml peripheral venous blood, and after cell washing and counting were distributed in wells with ESAT-6- and CFP-10-specific antigens as well as phytohaemagglutinin as positive control and cells only as negative control (2·5×105 cells/well) on a plate covered with anti-interferon-γ antibodies which were left to incubate overnight. After washing the plate a conjugate was added against the antibodies used, an enzyme substrate was also added.
The number of spots was determined using an automatic reader (AID ELISPOT, AIDSystem, Germany) as well as with visual assistance. The result was considered positive if the number of spots in any well with antigen was ⩾6 after subtracting the number of spots from the negative control. Negative results with ESAT-6 and CFP-10 antigens and phytohaemagglutinin were considered indeterminate.
Statistical analysis
The descriptive statistics were expressed as mean±s.d. for the quantitative variables and absolute frequencies and percentages for the qualitative variables. The comparisons were made by means of χ2 test and Student's t test. Kappa (κ) statistics were used to evaluate the agreement between the diagnostic tests. Values from P<0·05 were considered statistically significant. The data analyses were performed with SPSS software, version 11.5 (SPSS Inc., USA).
RESULTS
In total, 139 patients were admitted to detoxification between February 2006 and May 2007; the results of the interferon-γ release assays (IGRAs) were not valid in four cases. The mean (±s.d.) age of the 135 patients analysed was 39·8±8·0 years (83·7% males). The main drug abused was alcohol for 59 patients (45%), cocaine for 46 (35·1%) and opiates for 26 (19·9%). Sixty-one patients (45·2%) were current injecting drug users (IDUs); 42 patients (31·1%) tested positive for HIV infection upon admission; the characteristics of the patients with and without HIV infection are shown in Table 1. In HIV-positive patients the RNA-HIV was ⩾400 cp/ml in 38·1% of cases; 18 patients (42·9%) were undergoing antiretroviral treatment.
Table 1. Characteristics of the study population according to HIV serostatus
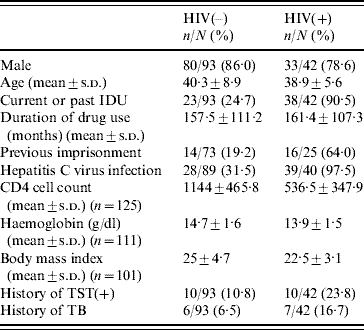
IDU; Injecting drug user; TST, tuberculin skin test.
In terms of history of TB, 13 patients (9·6%) had TB disease before admission and 20 (14·8%) had history of positive TST before admission. TST was performed on 100 patients and was positive in 29 patients globally (29%). Chest X-rays were performed for 113 patients and were normal in 96 (85%); eight patients (7·1%) presented with residual lesions and nine (7·9%) with findings attributable to chronic obstructive pulmonary disease.
Fifty-seven (42·2%) patients tested positive in at least one of the interferon-γ tests. With EIA, 46 patients (34·1%) tested positive, two were indeterminate (1·5%), and one was positive with ELISPOT and the other negative. ELISPOT was positive in 46 patients (34·1%) and indeterminate in one case, which was positive with EIA. The agreement between EIA and ELISPOT was 83% [κ=0·63, 95% confidence interval (CI) 0·50–0·76].
Prevalences of interferon-γ tests and TST in HIV-negative patients and HIV-positive patients with CD4 ⩾350 cells and <350 cells are shown in Table 2; there were no statistically significant associations between HIV serostatus and in vitro or in vivo tests.
Table 2. Prevalence of in vitro and in vivo tests for the diagnosis of latent tuberculosis according to HIV infection and CD4 cell count
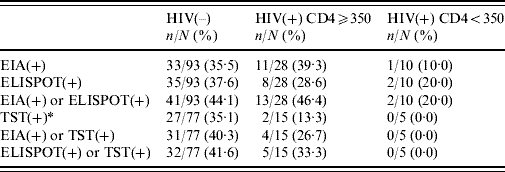
EIA, Enzyme immunoassay; ELISPOT, enzyme-linked immunospot technique; TST, tuberculin skin test.
* ⩾5 mm in HIV(+) patients; ⩾10 mm in HIV(–) patients.
Of the 100 patients given the TST test upon admission, the agreement between TST and EIA was 85% (κ=0·62, 95% CI 0·45–0·80) and was 83% between TST and ELISPOT (κ=0·57, 95% CI 0·39–0·75). Figure 1 shows the frequency distribution of the results from the two in vitro tests according to TST. Of the 29 patients who tested positive with TST, 20·7% (six cases) had negative results with IGRA. Of the 71 patients who tested negative with TST, 15·6% (11) tested positive with IGRA, four of which were HIV positive.
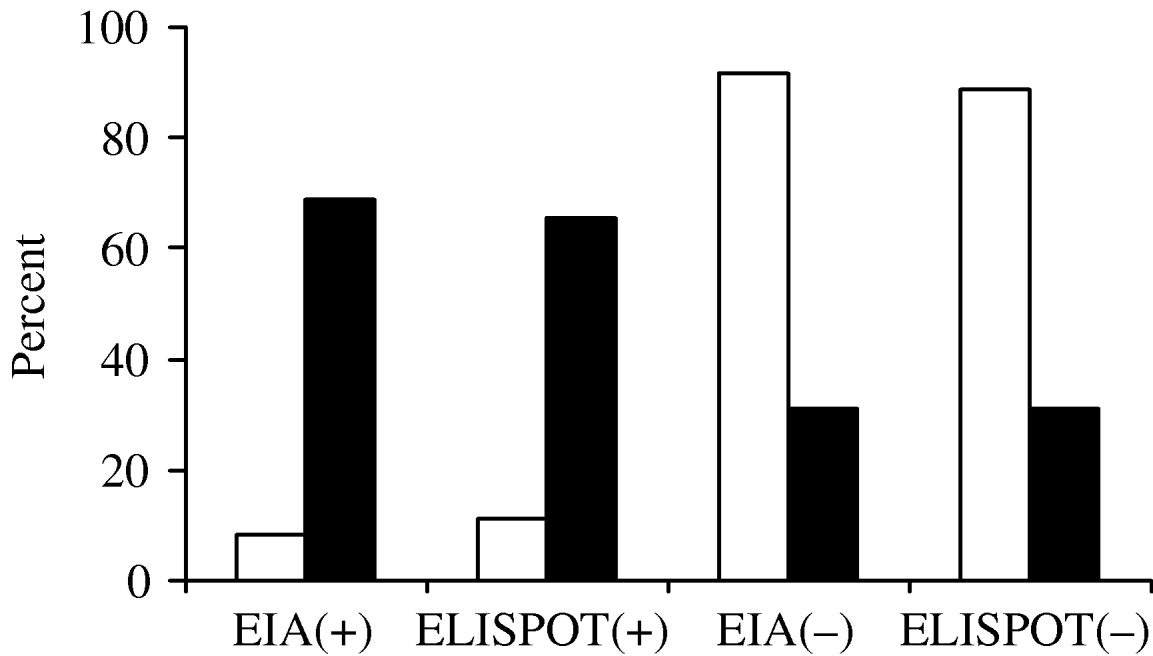
Fig. 1. Agreement of two interferon-γ release assays with tuberculin skin test (TST) at admission. □, TST(–); ▪, TST(+). EIA, Enzyme immunoassay; ELISPOT, enzyme-linked immunospot technique.
Table 3 shows the results of the interferon-γ tests in those patients with a history of TB disease. With EIA, positive results were obtained in 6/13 patients (46·1%) and six cases (46·1%) were also positive with ELISPOT with an agreement of 85%. Of those whose IGRA tests were positive, the time between TB disease and the interferon-γ test was 8·8 years for EIA and 9·8 years for ELISPOT compared to 14·7 years and 13·8 years for those whose EIA and ELISPOT interferon-γ results were negative.
Table 3. Characteristics of patients with previous tuberculosis disease and results of IGRA tests
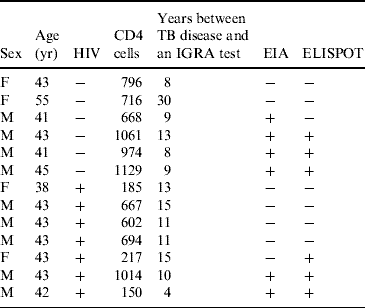
IGRA, Interferon-γ release assay; EIA, enzyme immunoassay; ELISPOT, enzyme-linked immunospot technique.
In the univariate analysis, age, sex, HIV infection, hepatitis C virus infection, previous imprisonment, being an IDU and the main type of drug were not associated with positive results with IGRA testing.
DISCUSSION
The results from this cross-sectional study show an elevated prevalence of latent TB in current alcohol and drug abusers independently of the in vitro method used. This fact is unsurprising since the level of TB rates in Spain have been high until recent years. In drug users, studies developed with the first generation of interferon-γ techniques that used stimulation with PPD found more than double the positive results in the in vitro test than in the skin test [Reference Converse13, Reference Kimura18]. Another study in patients receiving methadone showed a 17% prevalence of first-generation interferon-γ test [Reference Dewan19]. Only one study in drug addicts has used new interferon-γ assays with antigens specific for TB [Reference Grimes20]. To our knowledge this is the first study using IGRA testing in alcohol and drug abusers from Spain. The results described in a setting characterized by high prevalence of drug abuse and TB infection will allow better estimation of the burden of latent infection when using the new in vitro techniques. The observed agreement between the two in vitro techniques is good (κ=0·63) and coincides with that which has been observed in other populations [Reference Ferrara21].
The interferon-γ techniques that used TB-specific antigens have shown an elevated specificity (98% with EIA and 92% with ELISPOT) [Reference Pai, Riley and Colford14, Reference Liebeschuetz22] in low-risk populations; in terms of sensitivity, the results are controversial: while some studies find better sensitivity than in patients with TB disease [Reference Ferrara21–Reference Taggart23], in others, the sensitivity of EIA is less than that of TST for the diagnosis of TB [Reference Tsiouris24]. In immunocompromised subjects, ELISPOT has been associated with higher sensitivity than TST [Reference Pai, Riley and Colford14, Reference Menzies, Pai and Comstock16, Reference Liebeschuetz22, Reference Chapman25].
In the present study the agreement between TST and EIA and the agreement between TST and ELISPOT was 85% (κ=0·62) and 83% (κ=0·57), respectively. The discordance between IGRA and TST in the cases where TST was positive and IGRA negative could be ascribed to previous BCG vaccination, environmental mycobacteria, or an increased sensitivity of TST with respect to IGRAs. The cases where TST was negative and IGRA positive suggest higher sensitivity with new in vitro tests. However, the absence of a reference test for latent TB makes the evaluation of the sensitivity and specificity difficult to determine with IGRA.
The frequency of an IGRA test with indeterminate results is lower than observed in previous studies [Reference Ferrara21, Reference Brock26]. It is well known that indeterminate results are associated with immunodepression [Reference Menzies, Pai and Comstock16, Reference Rangaka27] and the association between the number of CD4 cells and indeterminate results [Reference Brock26]. The mean of CD4 cells in HIV-positive patients in this study was >500 cells/μl and only two of the 42 HIV-positive patients presented with CD4 counts <100 cells/μl, which would explain the low (with EIA) or null (with ELISPOT) number of indeterminate results. CD4 cell counts >500 cells/μl in HIV-positive patients might also explain the fact that no significant differences were observed for EIA and ELISPOT according to HIV serostatus at admission.
The results of the interferon-γ tests were positive in >50% of patients previously diagnosed and treated for TB disease. The same number of positive IGRA results (6/13) was obtained with the two tests in the subgroup that had TB disease before admission and the agreement between them was good. The duration of a positive IGRA test after TB (an average of 9 years in EIA-positive cases and 10 years in ELISPOT-positive cases), is even longer than that described in other studies [Reference Brock26]. In this sense, it is not well defined when interferon-γ tests become negative after treatment for TB; our results indicate that the IGRA test can remain positive for years after the illness which could limit its usefulness in differentiating current or past infection and should be an indication to perform additional tests for active TB.
We found no associations between a positive IGRA test and HIV infection, age, sex, substance of abuse, intravenous drug use or antecedent of imprisonment. Other studies in wider populations are necessary to assess the risk factors for TB infection when using new in vitro tests.
ACKNOWLEDGEMENTS
This work has been partially supported by grants from Ministry of Science and Innovation, Spain (FIS 07/0342, RTICS RD06/001/0021 and RD06/006/1014) and Fundació La Marató de TV3 (grant 02/1330).
DECLARATION OF INTEREST
None.








