Introduction
Urbanization of natural landscapes brings humans and their companion animals into contact with wildlife (Ordeñana et al., Reference Ordeñana, Crooks, Boydston, Fisher, Lyren and Siudyla2010), and they sometimes encroach on protected areas. Domestic dogs Canis familiaris pose distinct threats to wildlife. They harass wildlife and exhibit a surplus-killing behaviour (Kruuk & Snell, Reference Kruuk and Snell1981; Manor & Saltz, Reference Manor and Saltz2004; Banks & Bryant, Reference Banks and Bryant2007), compete for resources (Butler & du Toit, Reference Butler and du Toit2002; Butler et al., Reference Butler, du Toit and Bingham2004; Vanak et al., Reference Vanak, Thaker and Gompper2009) and spread diseases such as rabies, parvovirus and canine distemper (Cleaveland et al., Reference Cleaveland, Appel, Chalmers, Chillingworth, Kaare and Dye2000; Fiorello et al., Reference Fiorello, Noss and Deem2006; Vanak & Gompper, Reference Vanak and Gompper2009a). Dogs can also exert a top-down influence on smaller carnivores through interference competition or intraguild predation (Glen & Dickman, Reference Glen and Dickman2005; Mitchell & Banks, Reference Mitchell and Banks2005; Vanak & Gompper, Reference Vanak and Gompper2009b).
The effects of dogs on wildlife depend on their nature (domestic vs feral), on where they are found and on the factors controlling their numbers and use of space.
Domestic dogs are found in higher densities in areas with high human population density (Odell & Knight, Reference Odell and Knight2001; Ordeñana et al., Reference Ordeñana, Crooks, Boydston, Fisher, Lyren and Siudyla2010) and in rural areas where agricultural land borders nature reserves. The presence of domestic dogs in protected areas and their direct negative effects on native fauna are most significant at the borders, showing a decreasing trend from the anthropogenic matrix to the interior of the protected area (Torres & Prado, Reference Torres and Prado2010). Dogs could therefore exacerbate the negative anthropogenic edge effect associated with such border areas (Woodroffe & Ginsberg, Reference Woodroffe and Ginsberg1998; Revilla et al., Reference Revilla, Palomares and Delibes2001).
Feral dogs are completely wild and independent of humans (Nesbitt, Reference Nesbitt and Fox1975; Green & Gipson, Reference Green, Gipson, Hygnstrom, Timm and Larson1994), depending almost exclusively on wild-caught food (Glen & Dickman, Reference Glen and Dickman2005; Mitchell & Banks, Reference Mitchell and Banks2005). The direct threats of feral dogs to wildlife may therefore occur throughout entire protected areas.
In this context we studied the patterns of detection of dog tracks and the associated environmental and human constraints that could influence their presence in Doñana National Park, Spain, which has a high potential for the arrival and settlement of dogs, given its size and proximity to human settlements. We addressed two research questions: (1) Are dogs present in the Park? (2) What factors predict dog presence? We hypothesized that dogs using the Park could be either domestic dogs that enter occasionally from the surrounding matrix and are more abundant at the edges of the Park close to human settlements, or feral dogs that live and reproduce freely and are more evenly distributed throughout the Park, depending on habitat suitability and the availability of food. Doñana National Park is optimal for a study of this type because only part of its border is contiguous with human settlements and it is sufficiently large to potentially hold a feral dog population in its interior.
Study area
Doñana National Park is a flat sandy area located in south-west Spain (Fig. 1). We defined the anthropogenic edges of the Park as the northern and western edges, which are in close proximity to human settlements, crop fields and a highway, and the natural edges as the southern edge, along the Atlantic Ocean, and the eastern edge, along the Guadalquivir River (Fig. 1).
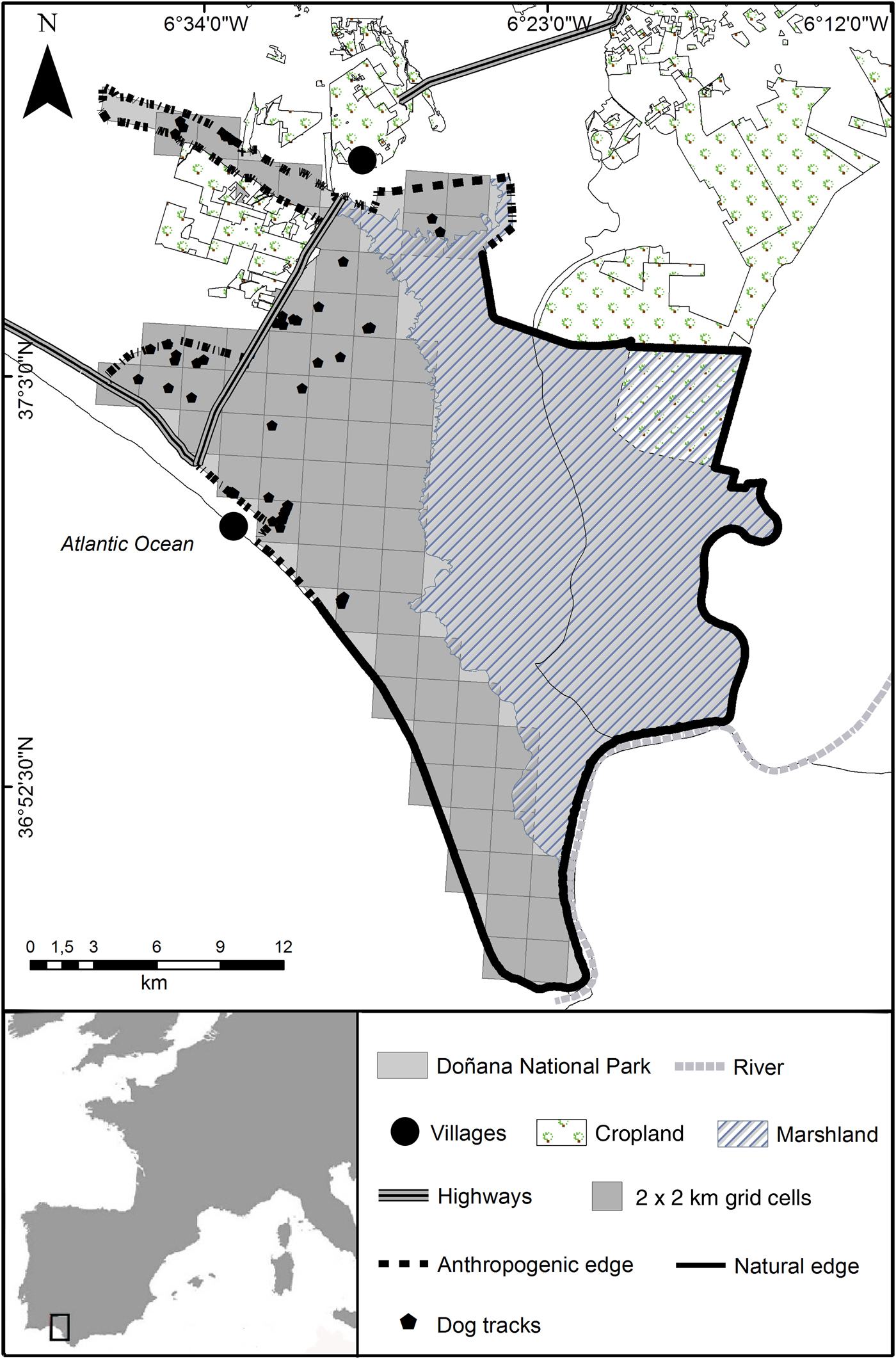
Fig. 1 Doñana National Park and the surrounding area. The locations where dog tracks were detected between November 2007 and April 2009 are shown. The rectangle on the inset indicates the location of the main map in southern Spain.
The suburban resort of Matalascañas, close to the western edge of the Park, has a fluctuating population between winter and summer, with c. 2,710 people in the summer. The village of El Rocío, situated close to the northern edge, has c. 1,635 residents year-round, although a spring pilgrimage brings up to one million visitors. There are also private farms nearby. The Park is fenced but the fence is permeable to small and medium-sized animals, including dogs.
The climate is Mediterranean sub-humid, with mild wet winters and hot dry summers, and mean annual rainfall of c. 550 mm. The Park's three main biotopes are scrubland, dunes and marsh (Valverde, Reference Valverde1958). Scrubland accounts for approximately half of the Park's surface area and is mainly characterized by heterogeneous patches of xerophytic plant species such as Halimium sp. and Cistus sp., and hydrophytic species such as Erica sp., with some patches of Juniperus phoenica and Pistacia lentiscus shrubs. Interspersed among the scrublands are scattered cork oak trees Quercus suber, wild olive trees Olea europaea, and patches of pine Pinus pinea and eucalyptus Eucalyptus sp. plantations.
Larger mammals in the Park include wild boar Sus scrofa, red deer Cervus elaphus and fallow deer Dama dama. Wild carnivores include red fox Vulpes vulpes, Eurasian badger Meles meles, Egyptian mongoose Herpestes ichneumon, common genet Genetta genetta, least weasel Mustela nivalis, European polecat Mustela putorius, Eurasian otter Lutra lutra, wild cat Felis silvestris and Iberian lynx Lynx pardinus. As well as 14 small to medium-sized mammal species, 397 bird species have been recorded, approximately half of which are breeding in the Park.
Methods
Surveys
We carried out dog-track surveys on sandy paths in 69 2 × 2 km grid cells located across the scrubland and dune areas of the Park during the wet seasons of 2007–2008 and 2008–2009. Surveys were carried out at least 3 days after rainfall and each track detected was geo-referenced using a global positioning system.
We searched for dog tracks in each cell by walking at least 3 km along sandy roads and fire breaks.
We assessed the environmental suitability of sampling cells to sustain a feral dog population, based on potential prey availability and general habitat structure. Feral dogs are habitat generalists and opportunistic foragers (Marsack & Greg, Reference Marsack and Greg1990; Boitani et al., Reference Boitani, Francisci, Ciucci, Andreoli and Serpell1995). Potential prey availability was estimated by counting tracks of small mammals, European rabbits Oryctolagus cuniculus, red-legged partridges Alectoris rufa, domestic cows Bos taurus and horses Equus caballus, and wild ungulates such as fallow deer, red deer and wild boar. Feral dogs hunt small prey and consume larger animals as carrion (Sillero-Zubiri & Macdonald, Reference Sillero-Zubiri and Macdonald1997; Butler et al., Reference Butler, du Toit and Bingham2004). We surveyed prey species by walking 7–10 25 m transects of c. 1.7 m width and separated by at least 300 m, within each 2 × 2 km cell. In the first year transect surveys of prey species were carried out throughout the wet season, when tracks from dogs were surveyed, but in the second year all transect surveys of prey species were carried out in April to avoid possible inter-monthly variations in abundance of some species (Kufner, Reference Kufner1986; Palomares et al., Reference Palomares, Delibes, Revilla, Calzada and Fedriani2001).
In the first year we recorded habitat structure in circles of 15 m radius around sampling points located every 300 m along the survey transects. We estimated visually the percentage of open ground cover and the percentage and modal height of three categories of vegetation: short shrubs (xerophytic species such as Halimium sp. and Cistus sp.), tall shrubs (Erica sp., J. phoenica and P. lentiscus), and trees. For each cell sampled we estimated the mean percentage cover at each sampling point.
Data analysis
We fitted generalized linear models with a binomial error distribution and a logit link function, in SAS v. 9.2 (SAS Institute, Cary, USA). We also incorporated methodological and climatic variables to control for their potential effects (Soto et al., Reference Soto, Desnica and Palomares2010).
Each grid cell was associated with a set of habitat variables, such as vegetation type (dunes, > 60% of open ground cover; pine forest, > 60% of pine vegetation; Mediterranean shrub, > 60% of short or tall shrub vegetation), and prey abundance (abundance index of total prey per km), and with a variable describing the location, based on the Euclidean distance from the centre of the cell to every infrastructure element; i.e. the fence close to human settlements (or anthropogenic edge of the Park), the fence without human settlements nearby (or natural edge of the Park), the nearest house or visitors’ centre, and the nearest paved road.
We used a two-step approach to analyse data. Firstly, we assessed which methodological and climatic variables potentially affect the likelihood of detecting dogs’ tracks and we selected the best-fitting model using an information-theoretic approach (Burnham & Anderson, Reference Burnham and Anderson2002). The models included the following variables: the observer who carried out the censuses, the relative humidity on census day (%), the number of days since the last rain, the year, and the maximum temperature (°C), calculated as the mean of the maximum temperature on the census day and the maximum temperature on two consecutive days before the census day. Climatic data were obtained from a meteorological station located inside the Park (Doñana Biological Station, 2009).
Secondly, we used this best-fitting model as a null model to develop a set of a priori models of detectability of dog tracks in the Park, based on three groups of hypotheses in relation to (1) the correlation between human presence and the presence of dog tracks (i.e. dogs being domestic), (2) dogs coming from a feral population (i.e. presence of dog tracks being related to environmental and/or prey variables), and (3) a combination of domestic and feral dogs. The variables included in the models were the minimum distance to the nearest house or visitors’ centre, the minimum distance to a human settlement, the distance to the nearest paved road, the distance to the anthropogenic edge of the Park, the distance to the natural edge of the Park, the abundance index of total prey per km, and the vegetation category. Among highly correlated variables (τ > 0.4), explored using Kendall's τ statistics, we retained the distance to the anthropogenic edge of the Park as the variable that best summarized the human influence.
We used the Akaike Information Criterion corrected for a small sample size (AICc) and calculated the difference in AICc between each model and the model with the lowest AICc (∆AICc; Burnham & Anderson, Reference Burnham and Anderson2002). The model with the lowest AICc and those with ∆AICc ≤ 2 were considered to be supported. ΔAICc values were used to compute Akaike weights (ω i ; Burnham & Anderson, Reference Burnham and Anderson2002). In addition, the relative importance of the predictor variable j(ω j ) was determined as the sum of ω i across all models where j occurred. Larger ω j values indicated a higher relative importance of variable j compared to other variables. For each hypothesis we used data from both years and we began by fitting all variables and then successively removed the terms that decreased the AIC most (Crawley, Reference Crawley2002).
Finally, we explored the classification accuracy of the selected models, using the nonparametric estimate of the area under the curve (AUC) of receiver operating characteristic plots (Hosmer & Lemeshow, Reference Hosmer and Lemeshow2000). AUC indices are in the range 0.5–1, with 0.5–0.7 indicating poor discrimination, 0.7–0.8 acceptable discrimination, 0.8–0.9 good discrimination, and > 0.9 outstanding discrimination. The area under the receiver operating characteristic curve is often used as a single threshold-independent measure for model performance and tests the ability of the model to discriminate between grid cells where dog tracks are present and those where tracks are absent (Fielding & Bell, Reference Fielding and Bell1997).
Results
Our surveys covered a total of 471 km and we recorded 72 observations of dog tracks (Fig. 1). We detected dog tracks in 16 grid cells in 2007–2008 and in 12 grid cells in 2008–2009.
We found a strong correlation between distance to the anthropogenic edge of the Park, distance to the natural edge of the Park, distance to the nearest village, and distance to the nearest paved road. Analyses were focused on the first two.
The best-fitting model explaining detection of dog tracks, based on non-biological factors, included humidity as a positive but non-significant predictor (odds ratio = 1.035, χ2 = 2.159, P = 0.142) and the number of days since the last rain as a negative and significant variable correlated with detection of dog tracks (odds ratio = 0.931, χ2 = 4.286, P = 0.035). Both predictors were therefore included as covariables in further analyses.
The analysis of detectability of dog tracks based on human-related, habitat and prey variables showed that the a priori hypothesis best adjusted to data included only human-related predictors. The best model for describing the detection of dog tracks in the Park, after adjusting for detection probability variables in the null model, included the distance to the anthropogenic edge of the Park (accounting for 27.6% of the deviance). The next model included the distance to the anthropogenic edge and the distance to the natural edge of the Park (models 1 and 3; Table 1). Detection of dogs was significantly and negatively associated with the distance to the anthropogenic edge of the Park (odds ratio = 0.737, χ2 = 8.020, P = 0.005; Fig. 2). The equation for this model (model 1; Table 1) is
where P is the probability of dog occurrence, D_ANT is the distance to the anthropogenic edge of the Park, Hum is humidity, and Rain is the number of days since the last rain. The relative importance of D_ANT was ω j = 0.999. The discriminating ability of the top model was AUC = 0.802 (P < 0.0001).
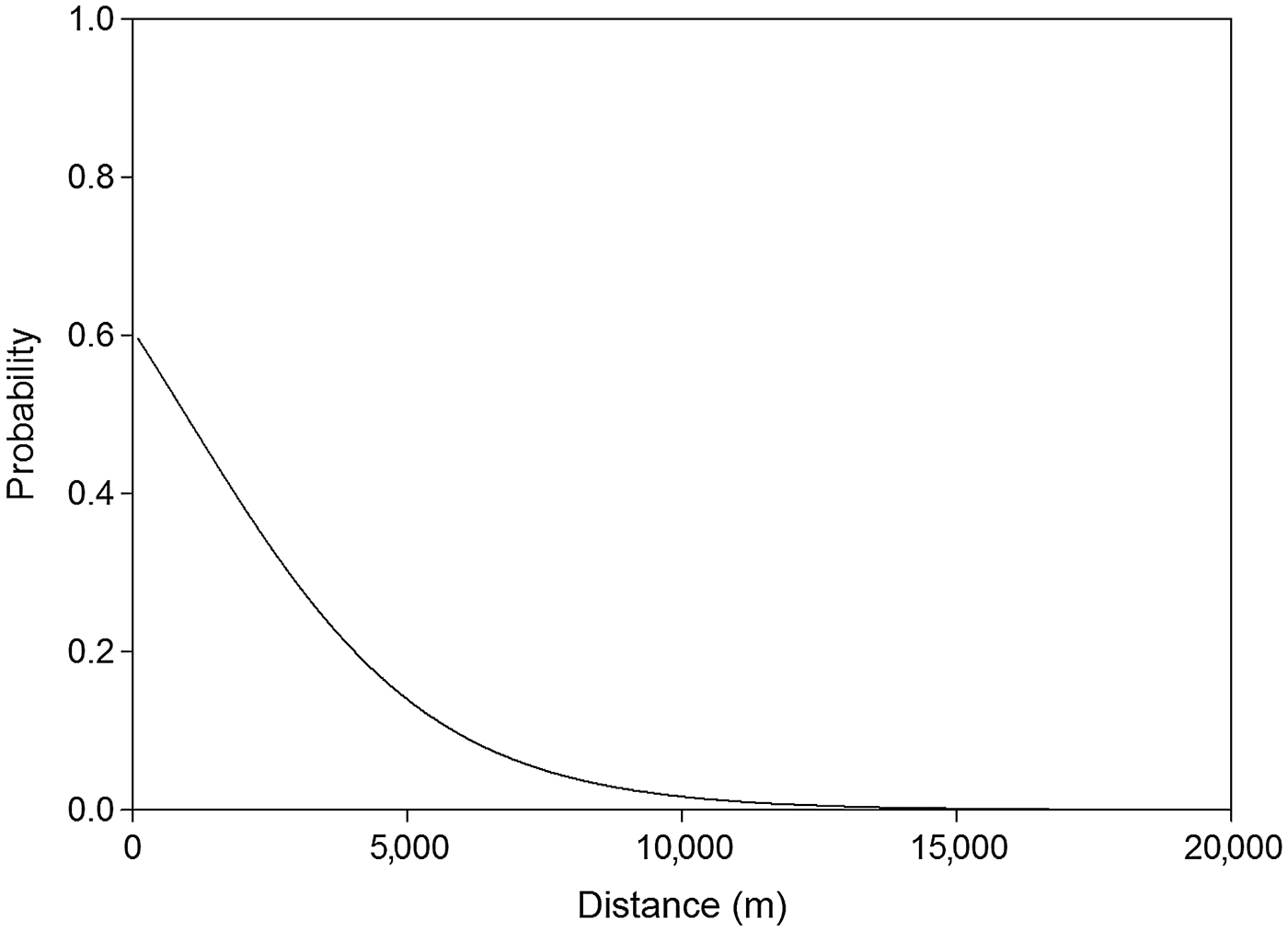
Fig. 2 Probability of detection of dog tracks as a function of distance to the anthropogenic edge of Doñana National Park (Fig. 1) during the wet seasons of 2007–2008 and 2008–2009.
Table 1 Logistic regression models used to investigate the effects of anthropogenic, habitat and a combination of all variables on detection of dog tracks at Doñana National Park (Fig. 1), with the model deviance, the sample-size-adjusted Akaike's information criterion (AICc), the difference in AICc value relative to the model with the lowest AICc (∆AICc), and the AICc weight.
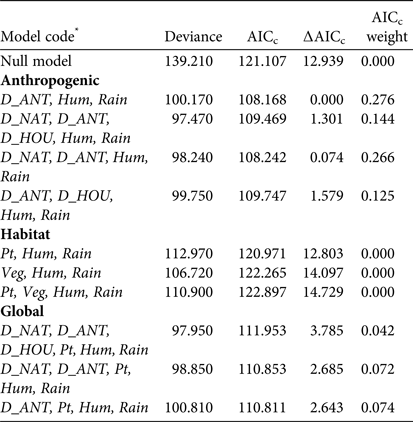
* D_ANT, distance to the anthropogenic edge of the Park; Hum, relative humidity; Rain, number of days since last rain; D_NAT, distance to the natural edge of the Park; D_HOU, distance to the nearest house or visitors’ centre; Pt, abundance index of total prey per km; Veg, vegetation category
Discussion
The detection of dog tracks in the Park was associated with distance from the anthropogenic boundary, a synthetic indicator of human influence that captures the effects of distance to the nearest village and the nearest paved road. We found many signs of dogs near the borders of the Park closer to households and we were unable to detect signs of dogs far from these anthropogenic edges. The detectability of dog tracks did not seem to be related to environmental variability such as vegetation type or prey availability. These findings support our hypothesis that dogs within the Park are domestic dogs that arrive occasionally from the surrounding matrix, not feral dogs living and reproducing freely. The lack of association between detection of dog tracks and availability of wild food resources suggests that dogs are dependent on humans for provision of food (Butler et al., Reference Butler, du Toit and Bingham2004; Vanak, Reference Vanak2008; Vanak & Gompper, Reference Vanak and Gompper2009b).
Although feral dogs survive and reproduce independently of human assistance, some feed on waste food discarded by humans (Green & Gipson, Reference Green, Gipson, Hygnstrom, Timm and Larson1994). Hence, a population of feral dogs within the Park could subsidize their diet in human settlements bordering the Park. Nevertheless, as the degree of reliance on humans distinguishes feral from domestic dogs, if dogs using the Park were from a feral population, living and reproducing freely but accessing human subsidies for food, we would expect dog detectability to be dependent on habitat suitability and/or the availability of wild food, and marginally dependent on human-related variables. Compared to domestic dogs, feral dogs are highly social, usually living in packs or groups (Daniels & Bekoff, Reference Daniels and Bekoff1989; Green & Gipson, Reference Green, Gipson, Hygnstrom, Timm and Larson1994), and we only detected isolated dog tracks. Camera-trapping studies conducted during the same period within the Park only detected dogs near human settlements, and all animals were identified as domestic based on their physical appearance (authors, pers. obs.).
The higher occurrence of dog tracks at the edges of the Park supports the idea that the presence of domestic dogs may be exacerbating the anthropogenic edge effect. Other authors have also reported a higher occurrence of domestic dogs near the edges of natural reserves compared to their interiors (Butler et al., Reference Butler, du Toit and Bingham2004; Srbek-Araujo & Chiarello, Reference Srbek-Araujo and Chiarello2008; Lacerda et al., Reference Lacerda, Tomas and Marinho-Filho2009; Marks & Duncan, Reference Marks and Duncan2009). Domestic dogs could therefore be considered a human-derived edge effect in protected areas, and wildlife may be most at risk of predation and displacement by dogs near the anthropogenic border. The higher occurrence of dogs in these border areas may exacerbate the existing edge effects on key population parameters (Murcia, Reference Murcia1995; Noss & Csuti, Reference Noss, Csuti, Meffe and Carroll1997), which cause the peripheries of reserves to function as population sinks (Woodroffe & Ginsberg, Reference Woodroffe and Ginsberg1998; Revilla et al., Reference Revilla, Palomares and Delibes2001).
Virulent, multi-host pathogens transmitted from dogs can cause mortality in wild animals and exacerbate the direct edge effects caused by domestic dogs in protected areas (Woodroffe & Ginsberg, Reference Woodroffe and Ginsberg1999; Cleaveland et al., Reference Cleaveland, Appel, Chalmers, Chillingworth, Kaare and Dye2000).
This represents a complicated scenario for conservationists, especially in areas with threatened endemic carnivore populations or where reserve size is small relative to species’ home ranges. More research is needed to help prevent or diminish the effects of domestic dogs on native fauna in protected areas. Transborder management actions must be prioritized in protected areas and we suggest that measures should include constraining the free-roaming of domestic dogs, raising awareness of responsible dog-ownership and biodiversity conservation (as some studies have indicated that dog interactions with prey species may be driven by hunger and inadequate diet; Sepúlveda et al., Reference Sepúlveda, Singer, Silva-Rodríguez, Stowhas and Pelican2014), removing un-owned dogs from reserve boundaries near human settlements, strengthening pet policies by forbidding unleashed dogs in public facilities, and imposing sanctions on owners whose dogs roam freely inside protected areas.
In the specific case of Doñana National Park, dogs living or spending time inside the protected area may pose a serious risk for the Iberian lynx (Ferreras et al., Reference Ferreras, Aldama, Beltrán and Delibes1992; Meli et al., Reference Meli, Cattoril, Martínez, López, Vargas and Simón2009; Millán et al., Reference Millán, Candela, Palomares, Cubero, Rodríguez and Barral2009), the most threatened felid species (Nowell & Jackson, Reference Nowell and Jackson1996). Hence, controlling the dog population at the Park is required for the conservation of one of the last meta-populations of the species. Free-roaming dogs are now prohibited from visitor facilities in the surroundings of the Park and, in light of the findings of this study, the Park's authorities are considering strengthening pet policies and developing campaigns of local awareness.
Acknowledgements
This study was financed by the projects CGL2004-00346/BOS (Spanish Ministry of Education and Science) and 17/2005 (Spanish Ministry of the Environment; National Parks Research Programme), and sponsored by Land-Rover España S.A. CS received a JAE pre-doctoral grant from CSIC (Spanish National Research Council). We are thankful to J.C. Rivilla and S. Desniça for their assistance during fieldwork, to N. Fernández for his help with data analysis, and to C. Dickman and P. Ferreras for their comments.
Biographical sketches
Carolina Soto is broadly interested in exploring how species select their habitats according to different ecological traits and inter-specific interactions between species in the same guild, and how that knowledge can be used to improve the management of populations of conservation concern. She aims to connect scientific research to decision tools and effective conservation management. She is also keen to research how evidence is used in conservation practice and evaluate the effectiveness of management plans for conservation of threatened species of mammalian carnivores. Francisco Palomares focuses on ecology, inter-specific interactions, predator–prey relationships and conservation of mammalian carnivores. In recent years he has mainly been researching large American felids, developing effective monitoring techniques using non-invasive methods and studying the patterns of coexistence between big cats.







