Article contents
Ecofriendly synthesis of ultra-small metal-doped SnO2 quantum dots
Published online by Cambridge University Press: 16 March 2015
Abstract
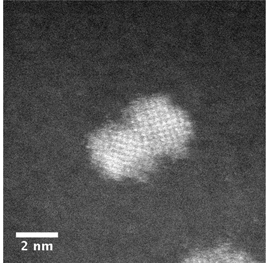
An ecofriendly synthesis is established to obtain ultra-small SnO2 nanoparticles (NPs) doped with metals by a hydrothermal method using only tin tetrachloride, urea, and water as reagents. This synthesis was done in a short period time at low temperature and without surfactants. Microscopy analysis revealed the formation of doped tin oxide NPs with a diameter smaller than 2.8 nm. Un-doped and doped tin oxides were obtained with a tetragonal type rutile structure with an average surface area of 348 m2/g.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2015
References
- 5
- Cited by




