Article contents
Improvement of catalytic activity and mechanistic analysis of transition metal ion doped nanoCeO2 by aqueous Rhodamine B degradation
Published online by Cambridge University Press: 04 September 2015
Abstract
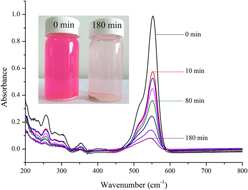
We compared the enhancement of photoactivity of transition metal ion (1 mol% Fe, Cu, Mn, and Zn) doped CeO2 nanocatalysts, and examined the effects of oxygen vacancies and the valence of the doped ions. The nanocatalysts were synthesized using a coprecipitation method and were characterized by x-ray diffraction, scanning electron microscopy, transmission electron microscopy, Brunauer–Emmett–Teller isotherm methods and Raman spectroscopy. The photocatalytic activities of these catalysts were tested using aqueous Rhodamine B (RhB) degradation under UV irradiation. The spherical CeO2 nanocatalysts had a mesoporous structure and ∼15 nm average particle size. The catalytic activity was closely related to the oxygen vacancies and the valence of the doped ions. An increase in oxygen vacancies of doped CeO2 decreased the photocatalytic activity. The photocatalytic activities of the catalysts decreased in the order: 1 mol% Fe > Cu > Mn > Zn > undoped CeO2. The 1 mol% Fe doped CeO2 degraded ∼92.6% of the RhB after 3 h of irradiation, and the degradation obeyed pseudo-first-order kinetics. Liquid chromatography–mass spectrometry indicated that the photodegradation of RhB was a stepwise oxidation process. Under continuous oxidation, over a long reaction time, the RhB was completely oxidized to its final products, such as water and carbon dioxide.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2015
References
REFERENCES
- 10
- Cited by




