Article contents
Modulated martensite formation behavior in Fe–Ni-based alloys; athermal and thermally activated mechanisms
Published online by Cambridge University Press: 30 June 2015
Abstract
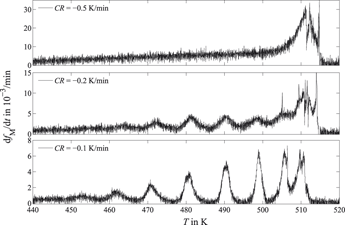
The martensitic transformation of Fe–22 wt% Ni austenite was investigated by high-resolution dilatometry as well as differential thermal analysis. Macroscopically discontinuous formation of lath martensite was observed, manifested in a train of transformation-rate maxima. It is proposed that the modulation of the transformation rate is caused by simultaneous formation of blocks in different martensite packages. The origin of simultaneity is ascribed to the interplay of chemical driving force, developing strain energy, and its relaxation upon sufficiently slow cooling. The transformation-rate maxima become more distinct with decreasing cooling rate (CR), clearly indicating the involvement of a thermally activated process in martensite formation. Quantitative analysis of the microstructure of differently cooled specimens revealed smaller martensite block sizes for higher CRs. All observations are compatible with athermal nucleation and thermally activated growth. (Local) strain relaxation in the austenite was identified as the involved thermally activated mechanism.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2015
References
REFERENCES
- 7
- Cited by




