Article contents
Cooling rate dependent undercooling of Bi in a Zn matrix by differential fast scanning calorimetry
Published online by Cambridge University Press: 17 December 2014
Abstract
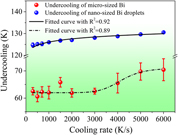
We presented the investigation on the cooling rate dependent undercooling of the microsized and nanosized Bi droplets in the Zn matrix via differential fast scanning calorimetry at scanning rates ranging from 300 to 6000 K/s. The experimental results demonstrated that the embedded nanosized Bi droplets gave more reproducible undercooling measurements than that of microsized Bi droplets at the grain boundaries. In addition, different cooling rate dependences of undercooling of microsized and nanosized Bi droplets were found. When the cooling rate is increased from 300 to 6000 K/s, the undercooling of the embedded nanosized Bi droplets increased gradually from 125 to 130 K. However, for microsized Bi droplets at the grain boundaries, there was an obvious increase of undercooling when the cooling rate was higher than 2000 K/s. In other words, the undercooling evolution displayed a sigmoidal relationship with the increase in cooling rate, indicating the change of the heterogeneous nucleation mechanism from a surface-induced mode to a volume-induced one.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2015
References
REFERENCES
- 6
- Cited by




