INTRODUCTION
Infection with high-risk human papillomavirus (HR-HPV) types is the major aetiological cause of cervical cancer and its pre-cancerous lesions [Reference Walboomers1], with types 16 and 18 accounting for ~70% of cervical cancers and ~50% of high-grade lesions [cervical intraepithelial neoplasia 2 and 3 (CIN 2/3)] [Reference Smith2]. Persistent HR-HPV infection is considered necessary to promote progression of pre-malignant stages to invasive cancer [Reference Moscicki3]. Compared to cervical cytology, cervical cancer screening by molecular HR-HPV testing is more sensitive, although less specific for the detection of CIN 2/3 [Reference Cuzick4].
Currently in the USA, HR-HPV testing is recommended and frequently used for co-testing with cytology for screening of women aged ⩾30 years (hereafter termed older women) [Reference Saslow5, Reference Moyer6]. Current guidelines do not recommend HR-HPV DNA testing in women aged <30 years (younger women), since HPV infection is relatively common in this age group and is a less specific predictor of high-grade cervical pre-cancer or cancer [Reference Schiffman7]. Examining the acquisition of cervical lesions in women worldwide, stratified by age and by baseline HPV testing results (overall or high-risk HPV), allows for a comparison of the difference in risk of the development of cervical lesions in older compared to younger women.
Although HPV vaccines are also licensed for use in women aged >26 years in many countries, the main target population for HPV vaccination is adolescent girls before the onset of sexual activity. In women aged 24–45 years, the quadrivalent HPV-16/-18/-6/-11 vaccine (Gardasil®, Merck & Co. Inc., USA) was efficacious in protecting against HPV-related outcomes in women not previously exposed to the HPV vaccine-types, but had limited protection in women who previously had a vaccine-type infection [Reference Muñoz8]. Initial results of the bivalent HPV-16/-18 AS04-adjuvanted vaccine (Cervarix®, GlaxoSmithKline Vaccines, UK) trial in women aged ⩾26 years also showed high vaccine efficacy against HPV-16/-18-associated CIN 1+ and/or persistent infection, but vaccine efficacy decreased when taking into account women with previous HPV infection or disease [Reference Skinner9]. Full results of the bivalent vaccine trial will be published shortly. However, as older women are still at risk of HPV infection and subsequent development of disease, examining patterns of cervical lesion development in women aged >26 years can provide valuable data on the burden of disease for the future development of cervical cancer prevention strategies for this age group.
Despite a relatively large number of studies which examined the incidence of cervical lesions stratified by HPV infection status and by age, no systematic review of the literature has been conducted to date. Thus, we present here a systematic review summarizing data on the incidence of cervical lesions in women with normal cervical cytology at baseline, stratified by population, age, follow-up time and baseline HPV infection status.
METHODS
Identification of eligible studies
Published studies were identified through PubMed (1 January 1989 to 30 September 2012) and the reference lists of eligible articles. No language restrictions were imposed. The following search terms were included: genital lesions, cervical cancer, cervical dysplasia, cervical lesions, squamous intraepithelial lesions (SIL), CIN, atypical squamous cells of undetermined significance (ASCUS), cohort study, and incidence. Only studies conducted in 1989 or later [after the implementation of the Bethesda System (TBS)] were considered in order to reduce the possibility of misclassification of cytological diagnoses.
To be eligible, a study must have reported at least population age and one estimate of the incidence of cytological or histological cervical abnormalities. Studies of HIV-seropositive populations, those with baseline prevalence of cervical abnormalities exceeding 15%, and those of women attending sexually transmitted diseases clinics were omitted as these women were considered at a relatively higher risk of incident cervical lesions than the general population. For HPV vaccine trials, only women in the control arm (unvaccinated population) were included in our analyses. All search results were independently reviewed (J.T. and B.W.) to ensure that no relevant articles were omitted.
Data abstraction
Abstracted data included (i) incidence proportion (equivalent to cumulative incidence) with associated standard errors or confidence intervals (CIs), and (ii) time interval over which incidence was measured. Study information abstracted included first author, publication journal and date, country and city of the study, period in which study was conducted, characteristics of study population (e.g. attendees of organized or routine cytological screening), mean, median or age range of study population, study exclusion criteria (e.g. current pregnancy, past hysterectomy), sample size (number of women screened), baseline cervical status, cervical cancer diagnostic method used at baseline, baseline HPV DNA or serology prevalence or type, HPV testing method [polymerase chain reaction (PCR) or hybrid capture 2 (HC2)], PCR primers if available (MY09/11, GP5+/6+, SPF-10, etc.), mean/median time of follow-up, and final diagnosis of cytological [ASCUS, low-grade squamous intraepithelial lesion (LSIL), or high-grade squamous intraepithelial lesion (HSIL)] and/or histological (CIN 1, 2 or 3) lesions. Abstracted data were double-checked by two independent investigators (H.S. and X.S.) and any discrepancies were resolved by consensus.
Selection of incidence estimates
Some articles that met the inclusion criteria were based on the same study population. To maintain the independence of study results, we chose the article with the greatest number of women in the incidence proportion analysis for cervical lesions, or the most recent study results, if study sample sizes did not vary. If multiple articles from the same study population could contribute to separate analyses (e.g. if one article provided incidence data by age, while another provided data by baseline HPV infection), all relevant articles were included. The time at which incidence proportion was measured differed across studies, so the corresponding length of follow-up was abstracted. For studies in which the estimated standard error for the incidence proportion was not reported, it was calculated as the square root of (p*(1 – p))/n, where p is the observed proportion of incident cases and n the sample size.
Descriptive analyses
If an article reported multiple results that fell into different categories of study characteristics, the article was included in all relevant categories in Table 1 so that the proportion of study results could add to more than 100%. Selected characteristics as well as the incidence proportions and their associated 95% CI for every study included in this review are listed in Tables 2a and 2b , and arranged according to region and by follow-up time. Figures of incidence proportion over time by age and baseline HPV infection are presented in Figures 1 and 2, and were generated using GraphPad Prism version 6.00 (GraphPad Software, USA). In addition, we also conducted meta-regression of incidence proportion of HSIL/CIN 2/3 in women with normal baseline cytology, stratified by baseline HPV infection (see Appendix).
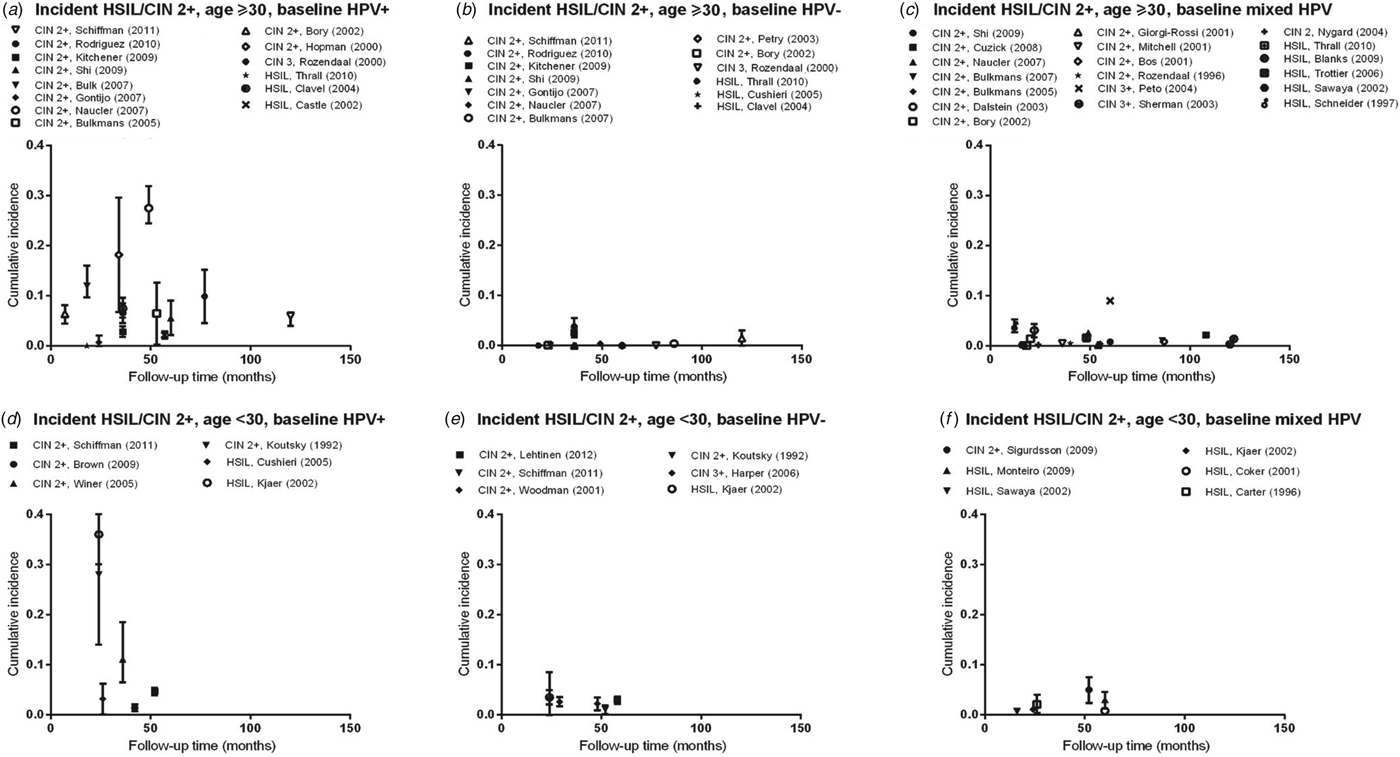
Fig. 1. Normal baseline cervical cytology to HSIL/CIN2/3 by age and HPV infection at baseline. CIN, Cervical intraepithelial neoplasia; HPV, human papillomavirus; mixed HPV, studies that include both HPV-positive and HPV-negative women; HSIL, high-grade squamous intraepithelial lesions.
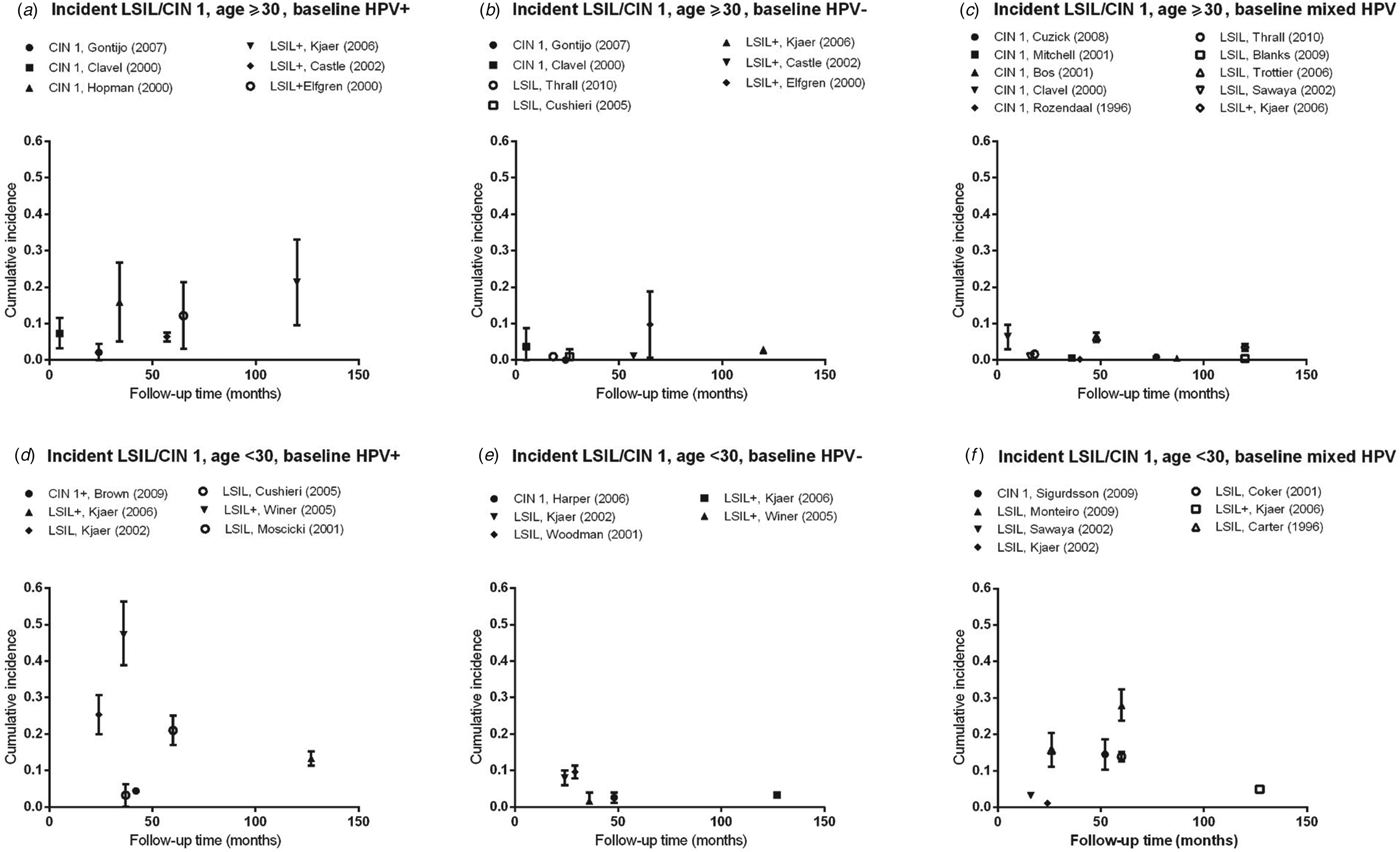
Fig. 2. Normal baseline cervical cytology to incident LSIL/CIN 1 by age and HPV infection at baseline. CIN, Cervical intraepithelial neoplasia; HPV, human papillomavirus; mixed HPV, studies that include both HPV-positive and HPV-negative women; LSIL, low-grade squamous intraepithelial lesions.
Table 1. Characteristics of studies on the incidence of cervical lesions
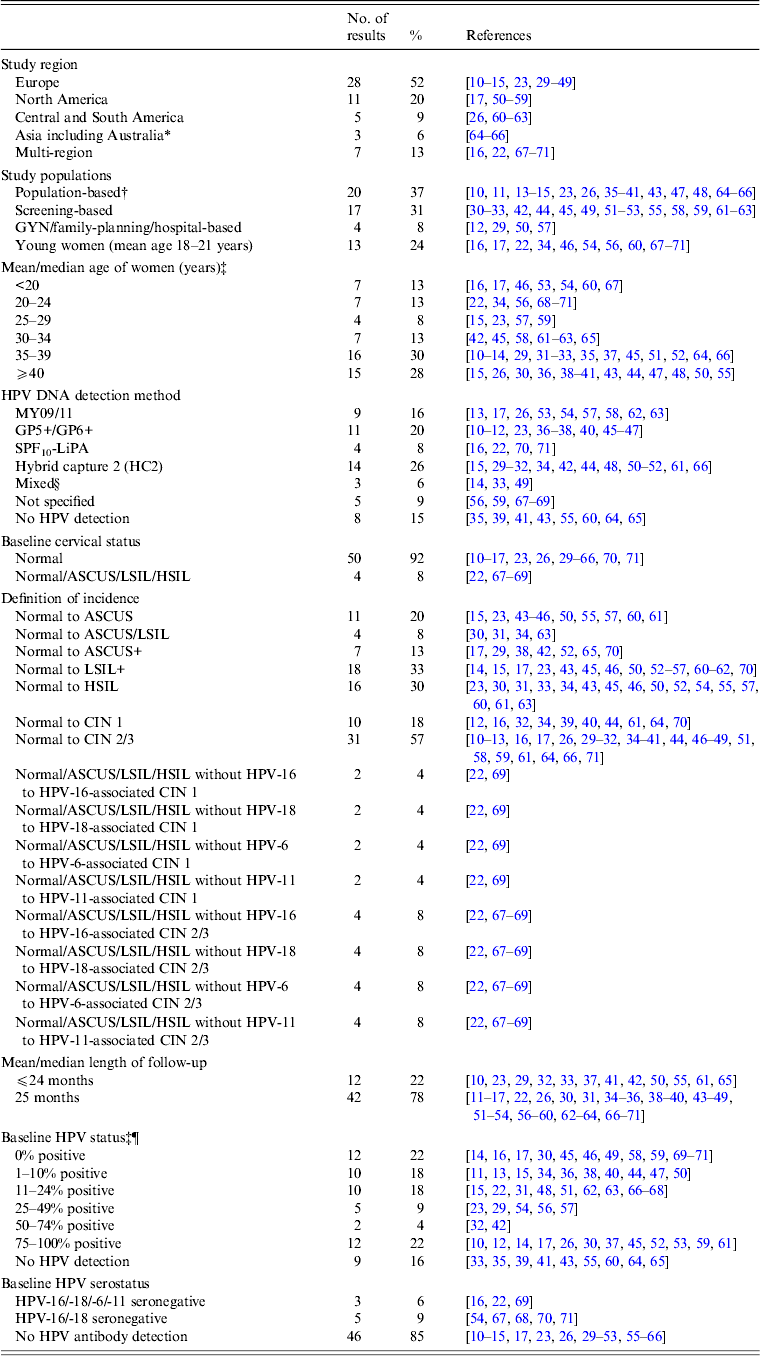
ASCUS, Atypical squamous cells of undetermined significance; CIN, cervical intraepithelial neoplasia; GYN, gynecological clinic; HPV, human papillomavirus; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion; NBCCEDP, National Breast and Cervical Cancer Early Detection Program.
* Includes two studies from Australia [Reference Mitchell64, Reference Mitchell and Medley65].
† Population-based studies from the following regions: Denmark [Reference Kjaer15, Reference Kjaer23]; Italy [Reference Giorgi-Rossi, Baiocchi and Ciatto35]; The Netherlands [Reference Bulk10, Reference Bulkmans36–Reference Nygard41, Reference Rozendaal47]; Norway [Reference Naucler11]; Sweden [Reference Elfgren14]; UK [Reference Peto13, Reference Blanks43, Reference Kitchener48]; Costa Rica [Reference Rodriguez26]; Australia [Reference Mitchell64, Reference Mitchell and Medley65] and China [Reference Shi66].
‡ Kjaer et al. [Reference Kjaer15] included a younger population (22–32 years) and an older population (40–50 years); Cushieri et al. [Reference Cuschieri45] included a population with baseline high-risk (HR)-HPV infection (median age 30 years) and a population without baseline HR-HPV infection (median age 38 years)
§ HPV DNA detection by HC2 followed by GP5+/6+ PCR [Reference Schneider33]; HPV DNA detection by MY09/11 and GP5+/6+ PCR [Reference Elfgren14].
¶ Elfgren et al. [Reference Elfgren14] included a population with baseline HPV DNA infection, age-matched to control population without baseline HPV DNA infection; analyses in Winer et al. [Reference Winer17] and Koutsky et al. [Reference Koutsky59] were stratified by baseline HPV DNA status; analyses in Clavel et al. [Reference Clavel30] were stratified by baseline HC2+ status.
Table 2(a). Selected characteristics of studies on incident high-grade intraepithelial lesions or cervical intraepithelial neoplasia 2 and 3
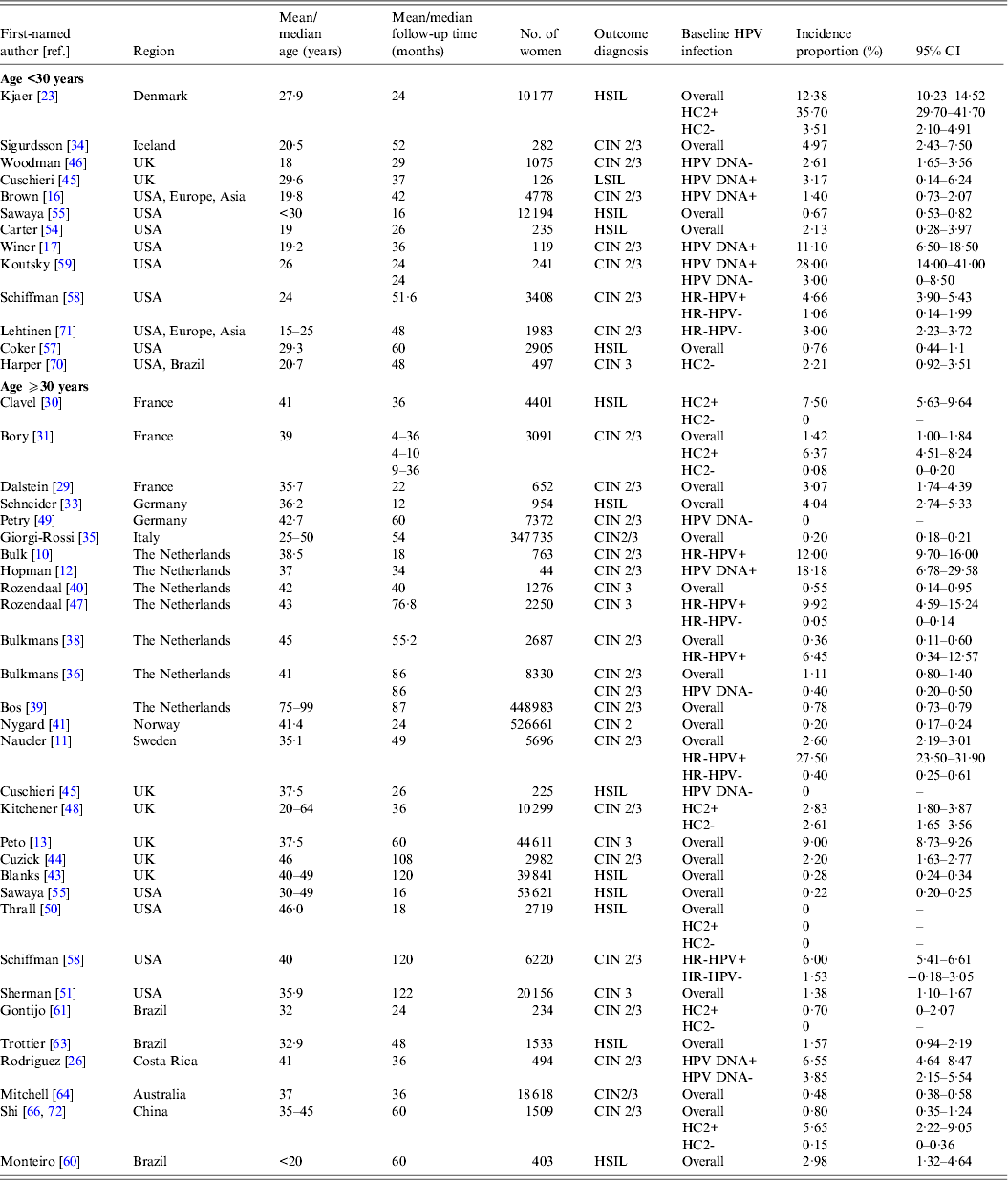
CI, Confidence interval; CIN, cervical intraepithelial neoplasia; HC2, hybrid capture 2; HR-HPV, high-risk human papillomavirus; HSIL, high-grade squamous intraepithelial lesions.
Table 2(b). Selected characteristics of studies on incident low-grade intraepithelial lesions or cervical intraepithelial neoplasia 1
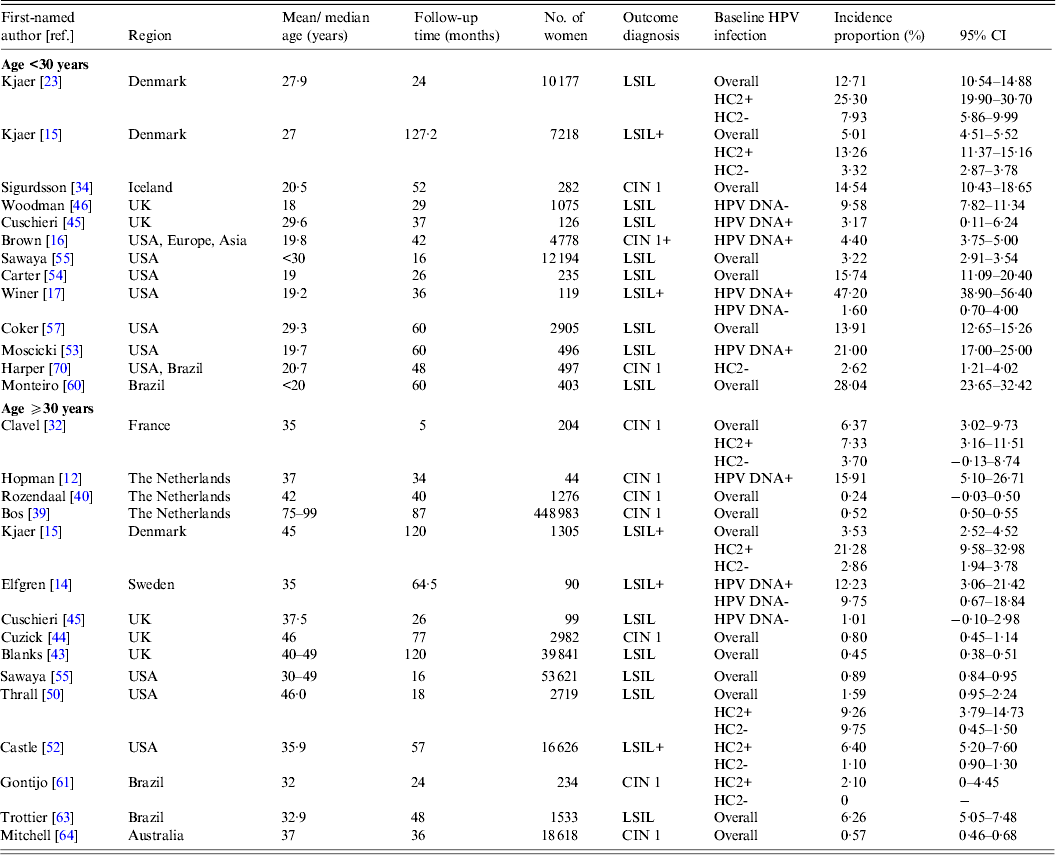
CI, Confidence interval; CIN, cervical intraepithelial neoplasia; HC2, hybrid capture 2; HR-HPV, high-risk human papillomavirus; HSIL, high-grade squamous intraepithelial lesions.
RESULTS
Eligible studies
Our systematic search of the published literature identified 54 studies that met the study inclusion criteria and reported non-duplicate results of the incidence of cervical abnormalities. Briefly, approximately half (52%) of the studies were conducted in Europe and 20% of the studies were conducted in North America (Table 1). Most of the studies were in populations of women with a mean or median age of ⩾30 years (71%).
Characteristics of abnormal cytology incidence and HPV testing
The majority (57%) of studies reported data on incidence proportion of CIN 2+ in women with baseline normal cytology. There are comparatively fewer studies reporting incidence proportion of CIN 1 from baseline normal cytology (18%). In terms of data on baseline HPV testing, approximately one-fifth (~21%) of the studies included only women who had an HPV infection, and another one-fifth (~19%) did not perform or report HPV testing. Baseline HPV serology was performed or reported in only 15% of included studies.
Cumulative incidence of HSIL/CIN 2/3
Estimates of incidence proportion for HSIL and CIN 2/3 (Fig. 1 a) were stratified by baseline HPV infection (HPV positive, HPV negative, or mixed (for studies that presented incidence proportion overall, without stratification by HPV infection), and further stratified by age group (<30 years, ⩾30 years). There was a greater number of studies with incidence proportion data for HSIL/CIN 2/3 in women aged ⩾30 years than for women aged <30 years.
In women aged ⩾30 years who were HPV positive at baseline, incidence proportion estimates for HSIL/CIN 2/3 generally increased with longer follow-up time (Fig. 1 a). Across studies, incidence proportion estimates varied at any given follow-up time. Nevertheless, across follow-up time (5–122 months), incidence proportion estimates were generally <10%, except in population-based screening in The Netherlands (mean age 38·5 years, incidence proportion of CIN 2/3 at 18 months = 12·0%) [Reference Bulk10], and in Sweden (mean age 35·1 years, incidence proportion of CIN 2/3 at 48 months = 27·5%) [Reference Naucler11], as well as for women in the pathology database of a hospital in Amsterdam, The Netherlands (median age 37 years; incidence proportion of CIN 2/3 at 34 months = 18·2%) [Reference Hopman12] (Table 2 a). In women aged ⩾30 years who were HPV negative at baseline (Fig. 1 b) or in mixed populations, incidence proportion estimates for HSIL/CIN 2/3 were generally <5%, remaining relatively constant across follow-up time (5–122 months) (Fig. 1 c), except in population-based screening in the UK (median age 37·5 years; mixed population, incidence proportion of CIN 3 at 60 months = 9·0%) [Reference Peto13].
Compared to women aged ⩾30 years, there were fewer data on the incidence of cervical lesions for women aged <30 years. Follow-up times available for women aged <30 years were also relatively shorter compared to those for women aged ⩾30 years (overall 16–60 months). In women with baseline HPV infection, incidence proportion estimates for HSIL/CIN 2/3 ranged from 1·4% (95% CI 1·0–1·8) to 35·7% (95% CI 29·7–41·7) over follow-up time between 24 and 60 months (Fig. 1 d). However, in women who were baseline HPV negative or in mixed populations, incidence proportion estimates for HSIL/CIN 2/3 were consistently ⩽5% over follow-up times of 24–52 months and 16–60 months, respectively (Fig. 1 e, f).
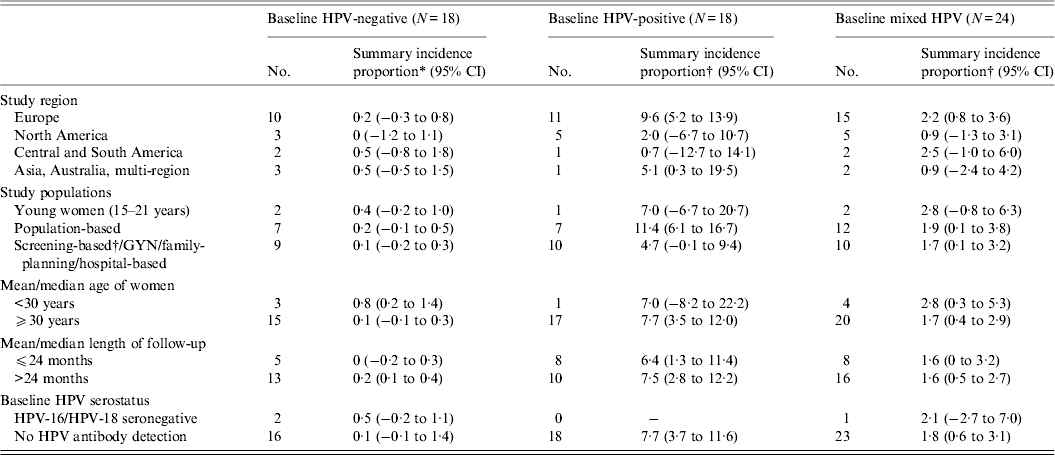
In our meta-analysis, the summary incidence proportion estimates of HSIL/CIN 2/3 were consistently higher across all variables in women with a baseline HPV infection, compared to mixed population and those without a baseline HPV infection (Appendix Table A1)., consistent with the data summarized in the figures. The summary estimates between different categories of a variable, however, did not reach statistical significance.
Cumulative incidence of LSIL/CIN 1
Estimates of incidence proportion for LSIL/CIN 1 were stratified by baseline HPV status (HPV positive, HPV negative, or mixed population) and further stratified by age group (<30 years, ⩾30 years) (Fig. 2). In women aged ⩾30 years with baseline HPV infection, incidence proportion estimates appeared to increase with longer follow-up time, although they were generally <10%, except in women in Amsterdam, The Netherlands (median age 37 years; incidence proportion of CIN 1 at 34 months = 15·9%) [Reference Hopman12], as well as in population-based screening in Sweden (median age 35 years; incidence proportion of LSIL+ at 65 months = 12·2%) [Reference Elfgren14] and in Denmark (median age 45 years; incidence proportion of LSIL+ at 120 months = 21·3%) [Reference Kjaer15] (Fig. 2 a, Table 2 a). In women aged ⩾30 years who were baseline HPV negative or in mixed populations, incidence proportion estimates were generally <5% over 5–120 months, remaining relatively constant across follow-up time (Fig. 2 b, c).
For women aged <30 years, incidence proportion estimates for LSIL/CIN 1 across follow-up time in women with baseline HPV infection were more variable than that for women aged ⩾30 years, ranging from 4·4% (95% CI 3·8–5·0) over 42 months [Reference Brown16] to 47·2% (95% CI 38·9–56·4) at 36 months [Reference Winer17] (Fig. 2 d). In women without baseline HPV infection, incidence proportion estimates were consistently <10% over follow-up time (24–127 months) (Fig. 2 e). In mixed populations, however, incidence proportion estimates for LSIL/CIN 1 appeared to increase with longer follow-up time, from 3·2% (95% CI 2·9–3·5) at 16 months to 28·0% (95% CI 23·7–32·4) at 60 months (Fig. 2 f).
DISCUSSION
This systematic review included data on over 2.2 million women from 54 studies to summarize the incidence of cervical lesions in women with normal baseline cervical cytology. Incidence of both high-grade (HSIL/CIN 2/3) and of low-grade (LSIL/CIN 1) lesions appeared to increase with longer study follow-up in women aged ⩾30 years with a baseline HPV infection compared to in women negative for HPV at baseline or in HPV-mixed populations within whom incidence remained relatively low. In women aged ⩾30 years with baseline HPV infection, the incidence of HSIL/CIN 2/3 did not appear to be notably higher than that of LSIL/CIN 1 in the same age group, and also did not appear higher than in young women aged <30 years with baseline HPV infection.
Our finding of a higher risk of HSIL/CIN 2/3 with longer follow-up time in women aged ⩾30 years with prevalent HPV infection, but not in women aged <30 years or in women without HPV at baseline, supports the current U.S. Prevention Services Task Force (USPSTF) recommendation for HPV co-testing with cytology of women aged ⩾30 years [Reference Moyer6]. In contrast, HPV infections detected in younger women at screening may represent newly acquired infections, the majority of which may be controlled and cleared and thus rarely lead to CIN 2/3 or cancer [Reference Brown16, Reference Wright18–Reference Rositch20].
The time from initial HPV infection to HSIL/CIN 2/3 can be short in some young women, consistent with results from the control arms of prophylactic vaccine trials [Reference Paavonen21, Reference Garland22]. In female university students aged 18–20 years in Seattle, USA, the overall incidence of CIN 2/3 was 11·1% at 36 months, and that of HPV-16- and HPV-18-associated CIN 2/3 was 27% at 36 months [Reference Winer17]. Similarly, another study in Denmark found a high incidence of HSIL (35·7% at 24 months) in young women from the general population (mean age 25 years) [Reference Kjaer23]. Although data on CIN 2/3 regression in these university students are not generally available given that cytological abnormalities were referred to colposcopy and treatment, if indicated [Reference Winer17], another study showed that most CIN 2 cases (~70%) in young women (mean age 20 years) regressed within 3 years [Reference Moscicki24]. Although our findings of non-negligible HSIL/CIN 2/3 incidence in women aged <30 years with prevalent HPV infection suggest potential benefit of HR-HPV DNA co-testing with Pap for some women aged <30 years, data on regression of HSIL/CIN 2/3 in young women are needed to determine the clinical significance of these lesions, particularly in terms of their potential to progress to cervical cancer [Reference Moyer6].
We found that in women aged ⩾30 years with baseline HPV infection, the incidence of HSIL/CIN 2/3 over follow-up time did not appear to be notably higher than that of LSIL/CIN 1. It is unclear from this literature review if these incident LSIL/CIN 1 cases were due to recently acquired or persistent HPV infections. Recent data have also found that most (85%) incident HPV infections in older women were detected during periods of sexual abstinence or monogamy, suggesting that the risk of newly detected HPV infections in older women are likely due to reactivation of latent infections [Reference Rositch25]. Previous longitudinal data from Costa Rica showed that new HPV infections in older women (⩾42 years) rarely progress to CIN 2/3 or cancer within 3 years, but longer follow-up of women is needed to understand their true risk of CIN2/3 development [Reference Rodriguez26].
We further identified nine eligible studies published between 30 September 2012 and 28 February 2014 [Reference Luyten75–Reference Sultana83], of which four were from Europe [Reference Luyten75–Reference Elfstrom78], four from the USA [Reference Wheeler79–Reference Castle82], and one from Australia [Reference Sultana83]. Six were population-based studies [Reference Luyten75–Reference Wheeler79, Reference Sultana83], and three were screening-based studies [Reference Katki80–Reference Castle82]. Total follow-up time ranged from 36 months [Reference Wheeler79] to 216 months [Reference Castle82]. Incidence proportion for HSIL/CIN 2/3 in women aged ⩾30 years with baseline HPV ranged from 0·7% (95% CI 0·14–1·27) over 36 months [Reference Wheeler79] to 7·0% (95% CI 5·7–8·6) over 216 months [Reference Castle82], and that for women without baseline HPV from 0% over 60 months [Reference Luyten75] to 1·4% (95% CI 1·2–1·7) over 216 months [Reference Castle82], consistent with our current results. However, in studies comparing women aged <30 and ⩾30 years [Reference Thomsen77, Reference Wheeler79], HSIL/CIN 2/3 incidence proportion overall appeared somewhat higher in women aged <30 years than in women aged ⩾30 years (3·8% vs. 1·3% over 126 months [Reference Thomsen77], and 0·4% vs. 0·1% over 36 months [Reference Wheeler79]).
In the USA, prophylactic HPV vaccines are licensed and recommended for women aged ⩽26 years [Reference Markowitz27, 28]. There is currently a lack of data suggesting high efficacy of vaccination in preventing infections or cervical lesions in women aged >26 years. Previous data on women aged 24–45 years showed that the quadrivalent HPV vaccine was efficacious in women with no evidence of previous exposure with the vaccine-types (defined by baseline seronegativity for HPV-6/-11/-16/-18 and DNA negative by PCR in cervical specimens), but not in women with prevalent HPV infections [Reference Muñoz8]. However, this clinical trial had a relatively short follow-up time (mean 2·2 years), and used an endpoint of combined incident HPV infection and cervical disease [Reference Muñoz8]. Further vaccine efficacy data on incident HPV infection and subsequent HSIL/CIN 2/3 development or regression in women aged >26 years with longer follow-up time will determine if relatively older women can potentially benefit from HPV vaccination, particularly those in whom prevalent HPV infection and cervical disease are no longer present.
It is important to note that incidence data of cervical lesions in women aged <30 years are generally limited (n = 18), compared to those in women aged ⩾30 years (n = 38). Data on follow-up time was also generally shorter for women aged <30 years than for women aged ⩾30 years. The lack of data on women aged <30 years limited our ability to draw definitive conclusions about the acquisition risk of cervical lesions over time, as well as about the benefits of HPV testing for cervical cancer screening in this younger age group. Further, we are unable to calculate and formally compare incidence rates, stratified by covariates such as prevalent HPV status and age, given that few studies reported person-time data. In studies where we could not determine the baseline HPV prevalence, we assumed HPV prevalence to be between >0% and <100% and thus included these studies in the ‘mixed’ HPV prevalence category. Data on the ‘mixed’ group were included so that we may observe the overall pattern of incident lesions over follow-up time in a given population, stratified by age. We also did not include data on the incidence of HPV type-specific associated lesions, given that studies with HPV genotype-specific results were few, and our study was initiated before the publication of the revised USPSTF screening guidelines in 2012 recommending HPV genotype-specific testing for HPV-16 or HPV-16/-18 for women who co-tested HPV positive and cytologically negative [Reference Saslow5, Reference Moyer6].
In conclusion, this systematic review showed that the incidence of HSIL/CIN 2/3 in women aged ⩾30 years is higher with prevalent baseline HPV infection, and increased with longer follow-up time. We found that some young women aged <30 years with prevalent HPV infection are at risk of developing HSIL/CIN 2/3 within a relatively short period of time, although we did not abstract data on the regression of these lesions to determine if they would be likely to advance to cancer. Our finding of a non-negligible increase in LSIL/CIN 1 over follow-up in women aged ⩾30 years who had a baseline HPV infection may indicate an HPV infection that is potentially progressing to higher grade cervical lesions. However, data on the potential of these LSIL/CIN 1 to progress to more severe cervical disease should be summarized to help inform cervical cancer prevention strategies.
APPENDIX
Meta-regression analyses
Standardization of estimates of incidence of cervical lesions
In the meta-regression analyses, length of follow-up was centred at 24 months. In cases where the incidence proportion of abnormal cytology was 0% or 100% and the standard error was undefined, the following adjustment was made to calculate the standard error: the overall meta-regression model was first fitted without study characteristics (intercept-only model) using all studies that did have a defined standard error [Reference Sweeting, Sutton and Lambert73]. This model produced a summary incidence proportion of HSIL/CIN2/3 of ~1% at 24 months. Consequently, the undefined standard errors were estimated by adding 0·01 to the number of women with incident HSIL/CIN 2/3 and 0·09 to the number of women without incident HSIL/CIN 2/3. Otherwise, if the standard error was not reported and could not be calculated from the information reported in the article, the incidence result was not included in the meta-regression analyses.
Meta-regression analyses
Meta-regression was used to produce summary estimates of the proportion of women who developed incident cervical abnormalities. When results from the same study population were included in different strata of an analysis, an indicator variable for that study population was included in the meta-regression model to account for the lack of independence. Meta-regression analysis was conducted in Stata version 10 (StataCorp, USA).
To formally compare incidence proportion across studies and by key study characteristics, random-effects meta-regression was used, with the among-study variance estimated by restricted maximum likelihood [Reference Thompson and Sharp74]. Stratified summary estimates allowed descriptive comparisons across individual categories of study characteristics by providing separate summary estimates and 95% confidence intervals for each category. Studies were allowed to contribute to more than one category to reduce any potential influence of the decision rules on the distribution of study and population characteristics.
Appendix Table A1. Meta-regression of the incidence proportion of HSIL/CIN 2/3 in women with normal cervical diagnoses at baseline, stratified by baseline HPV infection status
ACKNOWLEDGEMENTS
This study was funded by an unrestricted grant by GlaxoSmithKlineVaccines, Global Vaccine Development, Wavre, Belgium.
DECLARATION OF INTEREST
Jennifer S. Smith has received research grants, served on advisory boards, and/or been a speaker for GlaxoSmithKline, Merck Corporation, Hologic Gen-Probe, Qiagen, and BD Diagnostics. Sylvia M. Taylor is an employee of GlaxoSmithKline.











