INTRODUCTION
Globally, the incidence of pulmonary tuberculosis (PTB) has been slowly decreasing while an increase has been seen in the proportion of PTB cases with diabetes mellitus (DM). Meanwhile, prevalence of DM has continued to increase, especially in newly emerging developing countries where PTB is highly endemic [Reference Lönnroth1–Reference Shaw, Sicree and Zimmet3]. Research into the associations between DM and PTB has returned to the agenda [Reference Roglic and Unwin4–Reference Kim6]. The Fifth National Epidemiological Survey on PTB was completed at the end of 2010 in China and the results indicated that the prevalence of PTB at the national level showed a downward trend, compared to the Fourth National Survey in 2000. However, the declining rate was slow and the epidemic remained serious [7, Reference Wang8]. Following rapid economic growth, factors such as expansion of population, ageing, lifestyle shifts and urbanization have resulted in an increase in the prevalence of DM [Reference Alcorn and Ouyang9]. A recent study showed that total age-standardized DM prevalence was 9·7% in mainland China [Reference Yang10]. According to projections, the number of adults with DM has now reached 92·4 million in China. With the high burden caused by both diseases in China, the impact of DM on PTB may be considered as more serious compared to other countries. The World Health Organization (WHO) and the International Union Against Tuberculosis and Lung Disease (IUATLD) jointly proposed the ‘collaborative framework for care and control of tuberculosis and diabetes’, which comprehensively explored the relationships between PTB and DM. They concluded that DM was recognized as one of the risk factors for PTB. When patients had DM, the risk of contracting PTB [Reference Jeon and Murray5, Reference Kim6, Reference Pablos-Méndez, Blustein and Knirsch11–Reference Pérez, Brown and Restrepo15] was several times higher. However, findings on clinical presentation and treatment outcome of DM on PTB were more scarce and varied, with only a few studies presenting final treatment outcomes [Reference Ruslami16–Reference Alisjahbana18]. In China, little research has been conducted on the prevalence of DM in PTB [Reference Wang19], and there is a particular lack of research concerning the impact of DM on treatment outcome of PTB. This study was designed to explore the above issues in two districts of Beijing, in order to provide evidence for further large-scale prospective study and development of corresponding prevention and control strategies in the city.
METHODS
Study design and patient population
The Chinese Government issued the National TB Control Programme (NTP) for Tuberculosis Control and Treatment in 2001, which was a vehicle for the directly observed therapy, short course (DOTS) strategy. In order to implement the NTP, the China Ministry of Health published guidelines which involved a series of standards and standard operating procedures for PTB case detection and diagnosis, chemotherapy regimens and treatment management. As mentioned in the guidelines, there were corresponding levels of PTB dispensaries responsible for notification, diagnosis, treatment and management of PTB cases. In a prospective study, we included all registered PTB patients presenting at two PTB dispensaries in Beijing from January 2010 to December 2011, excluding subjects with incomplete medical records. According to the guidelines, all patients underwent extensive diagnostic testing to determine their TB status at the dispensaries once suspected of having PTB, which included sputum acid-fast bacilli (AFB) smear, purified protein derivative (PPD) skin test, chest radiograph (evaluated by two independent radiologists), clinical symptoms and signs. Drug susceptibility testing (DST) was performed for all re-treatment cases and cases of treatment failure. Multidrug-resistant tuberculosis (MDR-TB) was defined as tuberculosis that was resistant to at least isoniazid (INH) and rifampicin (RMP). All confirmed MDR-TB patients were transferred to hospitals for individualized MDR treatment. All PTB patients were screened for DM by measurement of fasting blood glucose (FBG) concentrations after investigating their history of diabetes. Cases with a history of DM were confirmed by diagnostic evidence (WHO diagnostic criterion for DM [20]) issued by hospitals under qualification. Cases without a history of DM but with FBG ⩾126 mg/dl (7·0 mmol/l) were referred to general hospitals for further confirmation by oral glucose tolerance test (OGTT) [20]. DM was confirmed if 2-h plasma glucose during OGTT was ⩾200 mg/dl (11·1 mmol/l) [20]. The test was performed as described by the WHO, using a glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water. Results of confirmation tests were released during the subsequent monthly follow-up visit. PTB patients with DM accepted oral anti-DM drugs as prescribed by doctors from general hospitals during the study. HIV test was not performed for PTB at the study sites. PTB treatment consisted of standard regimens according to guidelines for implementing the NTP. Regimens for new active PTB patients included 2H3R3Z3E3/4H3R3. Regimens for re-treated PTB patients included 2HRZES/6HRE or 2H3R3Z3E3S3/6H3R3E3. Informed consent forms were obtained from all subjects, and the study was approved by the ethical committee of Peking University Health Science Centre.
Characteristics and follow-up with patients
Information on demographic characteristics, clinical signs and symptoms, together with related information such as history of contact with PTB, HIV/AIDS infection, was recorded through questionnaires completed by trained doctors and healthcare workers before the initiation of PTB treatment. Patients receiving treatment included those who received initial treatment or were re-treated. Patients receiving initial treatment were defined as those meeting one of the following criteria: never taking anti-TB drugs for the purpose of PTB or irregularly taking anti-TB drugs for <1 month. Patients receiving re-treatment were defined as those meeting one of the following criteria: irregularly taking anti-TB drugs for ⩾1 month; initial treatment failure or relapse. A symptom score (0–10 points) was calculated on the basis of the following symptoms: coughing, expectoration, fever, haemoptysis, weakness, chest distress, chest pain, anorexia, night sweats and weight loss (1 point for each item). Patients with a symptom score ⩾4 were classified as having more symptoms. Findings from sputum smear examinatios (smear positive or negative), chest radiographs (pulmonary cavities present or not present) and number of symptoms were used as indicators to evaluate the severity of the disease. At least four visits by supervisors of the dispensaries were made with every PTB patient covering full courses of treatment, including two visits during the intensive phase and two visits during the continuation phase. Treatment response was evaluated through physical examination and microscopic examination at monthly follow-up visits. Community-based DOT was performed by doctors of village/community clinics who were also responsible for monitoring and reporting adverse outcomes [death due to TB or non-TB, lost to follow-up, withdrawal due to serious adverse drug reaction (ADR)] to the dispensaries. All treatment outcomes were investigated and registered by dispensary staff. Adherence was measured through personal interview and review of treatment records and was evaluated by doses taken, with >90% of doses taken as prescribed defined as regular treatment according to Chinese guidelines. Based on guidelines for implementing the NTP, specific definitions were developed to classify outcome of treatment, which in general, were divided into three categories: (A) Cured: sputum smear-positive PTB patients that completed the full course of treatment and showed two consecutive smear-negative results including one following the completion of therapy. (B) Completion of full-course treatment: PTB patients with sputum smear-negative results that completed the prescribed course of treatment and had a negative sputum smear microscopy result or did not receive a smear examination after completion of therapy; and sputum smear-positive PTB patients that completed the prescribed course of treatment, had negative sputum for the last sputum smear microscopy and did not receive a smear examination after completion of therapy. (C) Adverse outcome including treatment failure, lost to follow-up, withdrawal due to serious ADR, transferred to MDR treatment, died of PTB or non-PTB diseases, and other (refusal of treatment). Treatment failure of PTB was defined as a patient who was sputum smear positive at the end of the fifth month or after completion of anti-TB treatment. Participants were classified as lost to follow-up if they did not attend for review (scheduled or unscheduled) during the study period and could not be traced. Confirmed MDR-TB patients were transferred to hospital for individual MDR treatment. Death due to non-TB was defined as TB patients dying due to reasons other than TB, and death due to TB was defined as TB patients dying of TB. Of the three categories, A and B were considered to represent treatment success. Category C was defined as adverse outcome.
Data analysis and statistics
We compared findings for PTB patients with and without DM. Pearson's χ 2 and Fisher's exact tests were used to compare proportions and the Student's t test was used for normally distributed continuous variables. The non-parametric Mann–Whitney U test was used for non-normally distributed continuous variables. Univariate and multivariate logistic regression models were used to calculate odds ratios (ORs) for factors associated with clinical presentation. Age, gender and treatment classifications were included as independent variables in the logistic regression models. A two-sided P value <0·05 was considered significant for all analyses. The database was constructed with EpiData v. 3.1 (EpiData Association, Denmark) and data was analysed using SPSS v. 18 (SPSS Inc., USA).
RESULTS
Patient characteristics
One thousand, one hundred and twenty-six patients with PTB were selected from the two dispensaries after excluding 19 cases because of incomplete medical records (Table 1). Of the 1126 PTB patients, 182 (16·2%) were identified as having DM, including 164 self-reported cases with history of DM confirmed before PTB diagnosis. The other 18 new cases were diagnosed following confirmation of PTB. Comparing PTB patients with and without DM, those with DM were found to have the following characteristics: being older (72·5% vs. 24·7% in the >45 years age group, data not shown), and a higher percentage of cases under re-treatment (12·1% vs. 7·0%). No significant differences were found relating to gender and history of bacillus Calmette-Guérin (BCG) vaccination between patients with and without DM. No HIV positivity in PTB cases was reported during the study period.
Table 1. Characteristics of pulmonary tuberculosis patients with and without diabetes mellitus
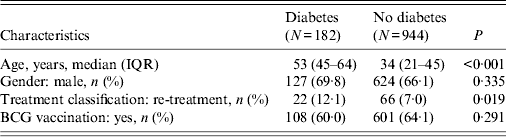
IQR, Interquartile range; BCG, bacillus Calmette-Guérin.
Disease presentations
Patients with DM had the following characteristics: higher proportion of smear-positivity (53·9% vs. 26·2%, see Table 2), higher proportion of pulmonary cavities (39·4% vs. 18·9%) and more symptoms (27·5% vs. 12·5%). Patients with DM presented with higher proportions on five related PTB symptoms: coughing, expectoration, haemoptysis, night sweats and weakness (P < 0·05). After adjusting for possible confounding variables such as age, gender and treatment classification, DM remained associated with presence of smear positivity (see Table 3), cavities and more symptoms.
Table 2. Disease presentation between pulmonary tuberculosis patients with and without diabetes mellitus
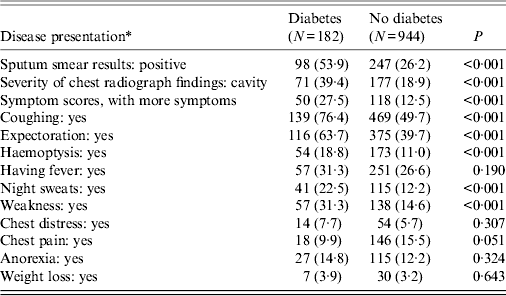
* All values represent the absolute number followed by the percentage in parentheses unless otherwise indicated.
Table 3. Related dependent variables at clinical presentation examined in multivariate regression analysis

OR, Odds ratio; CI, confidence interval.
* Adjusted for age (⩽45, >45 years), gender and treatment classification.
Patient treatment outcome
Data on final treatment outcome of all 1126 patients was collected at the end of 2012. There were 1066 (94·7%) cases recognized as having been successfully treated including 904 (95·8%) PTB patients without DM and 162 (89·0%) PTB patients with DM. All treatment outcomes of the two groups are listed in Table 4. Treatment adherences were estimated to be 98% in the group of patients with DM and 97% in the group of patients without DM. Results showed that PTB patients without DM had a significantly higher treatment success rate (P < 0·001). Further analysis showed that DM was associated with non-TB deaths (P < 0·001) and treatment failure (P = 0·002). Nine cases died of non-TB diseases in PTB patients with DM, including three that died of cancer, five of cardiovascular and cerebrovascular diseases and one that died of chronic bronchitis. There were also nine cases that died of non-TB diseases in PTB patients without DM, including five that died of cancer, three of cardiovascular and cerebrovascular diseases and one that died of renal failure.
Table 4. Treatment outcome of pulmonary tuberculosis (PTB) patients with and without diabetes mellitus
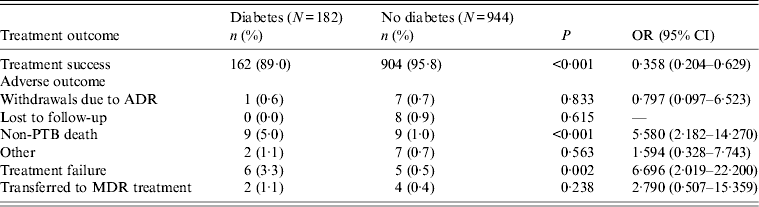
OR, Odds ratio; CI, confidence interval; ADR, adverse drug reaction; MDR, multidrug-resistant.
DISCUSSION
In our study, a significantly higher proportion of PTB patients with DM was discovered when comparing with results from previous studies in China [Reference Ruslami16, Reference Li21, Reference Zhang, Xiao and Sugawara22]. One of the explanations for the higher proportion was the higher DM prevalence in Beijing [Reference Yuan23, Reference Wang24]. This was even higher than the result found by a national study recently performed in China [Reference Alcorn and Ouyang9]. Recently, a community-based study was conducted by Wang et al. in China concerning screening for DM in newly detected PTB patients [Reference Wang19]. This study found that DM was observed in 6·3% of patients with PTB. In our study, the prevalence was almost three times (16·2%) that reported by Wang et al. The differences may be caused by different DM prevalence settings and different methods used for the diagnosis of DM. Our study was performed in districts of Beijing where reported prevalence of DM was over 10% in the general population [Reference Yuan23], while Wang et al.'s study was performed in rural area of Shandong province in north China where reported prevalence of DM was 4·7% [Reference Wang19]. DM was confirmed by OGTT after measurements of FBG in our study and confirmed by repeated measurements of FBG in the work of Wang et al. It was reported that OGTT was better than FBG in terms of both reliability and sensitivity [Reference Wallander25]. As mentioned in Wang et al.'s paper, DM prevalence may be underestimated due to the methods used for the diagnosis of DM. As reported previously that TB may be associated with increased blood glucose levels [Reference Jeon26], measurement of blood glucose at the time of PTB diagnosis may overestimate DM. In this study, the impact of overestimation may be slight because >90% of PTB patients with DM had already been diagnosed with DM before the PTB diagnosis was made. As in other studies [Reference Ruslami16], patients with both PTB and DM accounted for a higher proportion in the >45 years age group, almost three times that of PTB patients without DM. This phenomenon might be coincident with the peak age of DM. No gender difference was found between diabetic and non-diabetic patients [Reference Ruslami16]. No HIV/AIDS cases were reported in the study. As reported from the literature in 2013, the estimated epidemic of persons living with HIV/AIDS in Beijing remained low in China [Reference Ning27]. Patients with PTB and DM accounted for a higher proportion of patients classified in the ‘re-treatment’ category. This group accounted for nearly double the number of PTB patients without DM, indicating that DM may be associated with some adverse PTB treatment outcomes, such as treatment failure and relapse [Reference Ruslami16, Reference Baker17]. A higher proportion of smear-negative than smear-positive PTB cases was found in our study, which was aligned with the national information in the global TB report of 2012 [7]. PTB patients with DM had a greater likelihood of smear positivity, nearly twice that of PTB patients without DM. A strong association remained between smear positivity and DM after adjustment for possible confounding factors. Detection, treatment and management of smear-positive patients were recognized as key points for PTB control, implying that more attention needs to be paid to patients with both PTB and DM, given that more than half of smear-positive cases were in this group. Our findings showed higher frequency of cavities on chest radiographs in patients with DM, similar to findings from previous international studies [Reference Restrepo28, Reference Wang, Lee and Hsueh29]. Before the initiation of PTB treatment, patients with both PTB and DM presented more clinical symptoms, with five symptoms found to be statistically significant between TB patients with and without DM. While a paper from the Mexico–Texas border region reported that higher rates of fever and haemoptysis were seen in PTB patients with DM compared to those without [Reference Restrepo28]. These results suggest that PTB patients with DM not only presented PTB symptoms in general, but also ‘indicative’ PTB symptoms, which provided a basis for putting in place an early PTB screening programme for DM patients to check on both general and ‘indicative’ symptoms. Further analysis on differences of treatment success rate showed that DM was associated with a higher level of no-TB death and PTB treatment failure, a similar conclusion to that reached by a systematic review looking at the same problem [Reference Baker17]. This may be the reason that higher proportions of patients in the re-treatment category were found to be PTB patients with DM. However, the reasons for this remain unclear. The results of this study should, however, be interpreted in the context of certain limitations. First, the genotyping of diabetes was not involved thus impact of different diabetes classifications on clinical manifestations and treatment outcomes of PTB were not discussed. Second, PTB patients included in the study were selected from two TB dispensaries, lacking data from a small number of PTB patients who had received the full course of treatment in hospital. Third, the impact of DM could be evaluated only on PTB patients who could be diagnosed with TB. There was the possibility that DM patients with TB died before diagnosis thus underestimating the impact of DM on presentation and treatment outcome of PTB.
In summary, we saw a higher proportion of factors such as older age and PTB patients under re-treatment in PTB patients with DM than without. PTB with DM appears to be associated with a higher proportion of smear positivity, pulmonary cavities, more PTB symptoms, non-PTB death and treatment failure. DM probably had a negative impact on outcomes of PTB treatment. The findings of this study underline the need to perform early bi-directional screening programmes for PTB and DM in Beijing, and to improve care of patients with concomitant DM and PTB, especially those aged >45 years. Meanwhile, risk factors that promoted the development of active PTB in DM as well as related risks and mechanisms for different treatment response in PTB with DM need to be explored through prospective cohort studies.
ACKNOWLEDGEMENTS
We thank the following for their support: The PTB dispensary staff from the Changping and Fangshan districts in Beijing; and the staff from the National Center for Tuberculosis Control and Prevention, China CDC.
DECLARATION OF INTEREST
None.









