Most physiological functions decline progressively with age. Gastric acid secretion also decreases with advancing age in humans(Reference Borgström, Emås and Lilja1) and rats(Reference Maitra, Edgerton and Majumdar2, Reference Uchida, Misaki and Kawano3). Recently, Aoki et al. reported that chronic atrophic gastritis, a factor of hypochlorhydria, is very common among the elderly(Reference Aoki, Kihaile and Wenyuan4).
There are some studies suggesting the association between hypochlorhydria and bone loss. Many researchers have reported that gastrectomy induces bone loss in rats(Reference Dobrowolski, Piersiak and Surve5–Reference Shiga, Nishimukai and Tomita8) and human subjects(Reference Baek, Jeon and Lee9–Reference Zittel, Zeeb and Maier12). However, gastrectomy in itself has multiple risk factors other than hypochlorhydria for bone loss. For example, the production of the stomach hormone ghrelin, which is involved in bone physiology, is suppressed by gastrectomy(Reference van der Velde, Delhanty and van der Eerden13). Some studies have shown that the use of proton pump inhibitors (PPI), the most potent acid-suppressing drugs, is associated with an increased risk of hip fracture(Reference Yang, Lewis and Epstein14, Reference Vestergaard, Rejnmark and Mosekilde15). However, there is limited research showing the effects of hypochlorhydria induced by PPI on bone metabolism in human subjects or animals.
The absorbability of Ca is dependent on its solubility. The acidity in the stomach and resulting chyme increases Ca solubility(Reference Recker16). Thus, hypochlorhydria is considered to have a potential adverse effect on bone metabolism via the inhibition of Ca absorption.
The usefulness of cows' milk(Reference Opotowsky and Bilezikian17, Reference Teegarden, Lyle and Proulx18) or dairy products including cows' milk and yogurt(Reference McCabe, Martin and McCabe19) for the prevention of osteoporosis has been reported. Dairy products contain much soluble Ca and especially a dairy product fermented by lactobacilli (DFL) such as yogurt contains much l-lactic acid, which might result in the improvement of Ca solubility under hypochlorhydria. Therefore, we hypothesised that DFL may improve the effects of hypochlorhydria on bone metabolism.
In the present study, we indicated that PPI-induced hypochlorhydria decreases bone mineral density (BMD) in growing rats, which is accompanied by high bone turnover induced by secondary hyperparathyroidism due to Ca malabsorption. Additionally, we demonstrated that DFL intake cancels these adverse effects, probably via improving Ca malabsorption.
Experimental methods
Diets
The DFL was produced as follows: skimmed milk was cultured with Lactobacillus bulgaricus and Streptococcus thermophilus; to this, rennet containing chymosin (EC 3.4.23.4) was added. The DFL was separated from whey using a separator and subsequently lyophilised. The lyophilised DFL was sterilised by 40 kGy electron beam irradiation. We removed whey from the cultured skimmed milk to reduce the mineral content of the DFL.
The control diet was the modified American Institute of Nutrition (AIN)-93G diet with dextrin and sucrose as the carbohydrate source and casein as the protein source (Table 1). For the DFL diet, we substituted lyophilised DFL for casein; the l-lactic acid content of the DFL diet was 14·0 g/kg diet. Ca, P and crude protein were adjusted to the same level in the two experimental diets. Crude protein in the diets was calculated as total Kjeldahl N × 6·38.
Table 1 Composition of the experimental diets
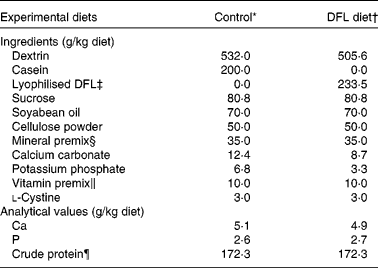
DFL, dairy product fermented by lactobacilli; AIN, American Institute of Nutrition.
* The modified AIN-93G diet.
† Containing a lyophilised DFL; the l-lactic acid content of the DFL diet was 14·0 g/kg diet.
‡ For details of production of the lyophilised DFL, see Experimental methods.
§ The mineral premix was prepared according to AIN-93G formulation(Reference Reeves, Nielsen and Fahey39) without Ca and P. It contained (g/kg): potassium citrate tri-potassium monohydrate, 70·78; sodium chloride, 74·00; potassium sulfate, 46·60; magnesium oxide, 24·00; ferric citrate, 6·06; zinc carbonate, 1·65; manganous carbonate, 0·63; cupric carbonate, 0·30; potassium iodate, 0·01; sodium selenate anhydrous, 0·01 025; ammonium paramolybdate 4-hydrate, 0·00 795; sodium metasilicate 9-hydrate, 1·45; chromium potassium sulfate 12-hydrate, 0·275; lithium chloride, 0·0174; boric acid, 0·0815; sodium fluoride, 0·0635; nickel carbonate, 0·0318; ammonium vanadate, 0·0066; powdered sucrose, 774·026.
∥ The vitamin premix was prepared according to the AIN-93 formulation(Reference Reeves, Nielsen and Fahey39) with choline bitartrate. It contained (g/kg): nicotinic acid, 3·000; calcium pantothenate, 1·600; pyridoxine-HCl, 0·700; thiamin-HCl, 0·600; riboflavin, 0·600; folic acid, 0·200; d-biotin, 0·020; cyanocobalamin, 2·500; all-rac-α-tocopheryl acetate, 15·000; all-trans-retinyl palmitate, 0·800; cholecalciferol, 0·250; phylloquinone, 0·075; choline bitartrate, 250·000; powdered sucrose, 724·655.
¶ Crude protein in the diets was calculated as total Kjeldahl N × 6·38.
Animals
A total of forty-eight Sprague–Dawley rats, aged 3 weeks, were purchased from Charles River Japan (Kanagawa, Japan) and cared for in accordance with the guidelines of the ethics committee on animal use of Meiji Dairies Corporation, as well as the relevant laws (no. 105, 1973) and notifications (no. 6, 1980) of the Japanese Government. The rats were individually housed in stainless-steel metabolism cages in a temperature-, humidity- and light-controlled room (21 ± 2°C, 55 ± 15 % humidity, 12 h light–12 h dark cycle). We used sixteen rats to investigate the effects of subcutaneous injection with omeprazole (OM) sodium (Omepral Injection 20; AstraZeneka, Osaka, Japan), a PPI, at a dose of 20 mg/kg on gastric acid secretion. After an adaptation period of 7 d, rats were divided into four body weight-matched groups of four rats. We measured the volume and pH of gastric juice according to the modified method of Uchida et al. (Reference Uchida, Misaki and Kawano3). Briefly, before and at 2, 9 and 21 h after subcutaneous administration with OM sodium at a dose of 20 mg/kg, the pylorus of the stomach of each rat was ligated and the abdominal incision was sutured under isoflurane anaesthesia. Gastric juice was collected for 3 h after the pylorus ligation. The gastric juice was centrifuged at 3000 g for 15 min, and the volume and pH of cumulative gastric juice were measured. All rats were fasted for 24 h before the operation.
We divided thirty-two residual rats into two body weight-matched groups of sixteen rats each: the control and DFL groups. All groups were allowed free access to their respective experimental diets and demineralised water for 9 d. On day 5 of the feeding period, each dietary group was subdivided into two body weight-matched groups of eight rats each: one received physiological saline injections (PPI − ) and one received PPI injections (PPI+). The PPI+ groups were subcutaneously injected with OM sodium at a dose of 20 mg/kg every 12 h from day 5 for 5 d. The PPI − groups were subcutaneously injected with physiological saline in the same manner. We measured food intake and body weight daily during the experimental period.
Sample collections
All faeces and urine of each rat were collected from day 6 for 4 d. After being cleaned of foreign adhering matter, the faeces were lyophilised and weighed. The lyophilised faeces were ground to a fine powder form. The faecal and urinary samples were used for Ca and P determinations.
Blood samples were obtained from the abdominal aorta using heparinised plastic syringes and needles under diethyl ether anaesthesia at the end of the experimental period. The plasma samples were separated by centrifugation at 3000 g for 15 min at 4°C and stored at − 80°C until analysis.
After killing, each rat's left and right femurs were excised and cleaned of adhering tissues. The right femurs were stored in a 70 % ethanol solution (Wako Pure Chemical Industries, Osaka, Japan) for X-ray computerised tomography (CT) measurement and mechanical testing. The left femurs were dried in an air-forced oven at 98°C and weighed. Subsequently, the left femurs were ashed in a muffle at 550°C and reweighed.
Determination of calcium and phosphorus concentrations
The samples of faeces, urine and plasma were mineralised in trace-element-grade concentrated nitric acid (Wako) using a microwave system (Multiwave3000; Perkin Elmer, Tokyo, Japan) and analysed for Ca and P by inductively coupled plasma spectroscopy (ICP-S7500; Shimadzu, Kyoto, Japan). The ashed femurs were completely dissolved in 1 m-nitric acid, diluted with demineralised water to the appropriate concentration, and used for Ca and P determinations by inductively coupled plasma spectroscopy.
Calculations
The amount of apparent Ca absorption (mg/4 d) was calculated as (Ca intake − faecal Ca) and the rate of apparent Ca absorption (% of intake) as ((Ca intake − faecal Ca)/Ca intake) × 100. The amount of Ca retention (mg/4 d) was calculated as (apparent Ca absorption – urinary Ca) and the rate of Ca retention (% of intake) as ((apparent Ca absorption – urinary Ca)/Ca intake) × 100. The parameters of P were calculated in the same way.
Determination of bone mineral density and bone strength by X-ray computerised tomography
The whole right femurs were scanned at 1 mm intervals using a LaTheta (LCT-100M) experimental animal CT system (ALOKA, Tokyo, Japan). The cortical BMD, cancellous BMD, total BMD, minimum moment of inertia of cross-sectional areas (MMICA) and polar moment of inertia of cross-sectional areas (PMICA) were calculated using LaTheta software (version 1.31; ALOKA, Tokyo, Japan). MMICA and PMICA represent the flexural rigidity and the torsional rigidity, respectively.
Mechanical testing
A three-point bending test was performed using a load tester (Bone Strength Tester model TK-252C; Muromachi Kikai, Tokyo, Japan) with the right femurs after scanning using the LaTheta experimental animal CT system. The right femurs were placed on a supporter with two loading points 4 mm apart. A breaking force was applied vertically to the midpoint of femurs by the crosshead at a constant speed of 5 mm/min until fracture occurred. The breaking energy of the femur was obtained from the load-deformation curve, which was recorded continually by a computerised monitor linked to the load tester.
Biochemical analysis
Plasma levels of osteocalcin, type I collagen C-telopeptides, 25-hydroxyvitamin D (25(OH)D) and 1,25-dihydroxyvitamin D (1,25(OH)2D) were assayed by a Rat-MID Osteocalcin EIA kit, a RatLaps EIA kit, a 25-Hydroxy Vitamin D EIA kit and a 1,25-Dihydroxy Vitamin D EIA kit, respectively, all of which were purchased from Immunodiagnostic Systems Nordic a/s (Herlev, Denmark). The plasma level of intact parathyroid hormone (PTH) was assayed by a Rat BioActive Intact PTH ELISA kit (Immnunotopics, San Clementre, CA, USA).
Statistics
Data were expressed as mean values with their standard errors. One-way ANOVA, followed by Dunnett's test, was used to analyse the effects of PPI administration on the volume and pH of cumulative gastric juice. Treatment effects were analysed by two-way ANOVA (diet × PPI), and Tukey–Kramer's test was used to detect significant differences among the groups. Differences were considered significant at P < 0·05. All statistical analysis was performed with StatView 5.0 (SAS Institute, Inc., Cary, NC, USA).
Results
Volume and pH value of cumulative gastric juice before and after proton pump inhibitor administration
The volume of cumulative gastric juice was significantly lower at 2–5 and 9–12 h after PPI administration compared with the basal level, and recovered to the basal level at 21–24 h after the administration. The pH value was significantly higher at 2–5 h after the administration compared with the basal level and recovered to the basal level at 9–12 and 21–24 h after the administration (Table 2). Ayazi et al. (Reference Ayazi, Leers and Oezcelik20) reported that the median gastric pH for normal subjects was 1·5 and classified the patients of gastric pH above the 95th percentile of normal (above pH 2·9) as hypochlorhydric. In the present study, the pH values at 0 h (basal) and 2–5 h after PPI administration corresponded with the normal and hypochlorhydric levels, respectively.
Table 2 Volume and pH of cumulative gastric juice before and after subcutaneous administration with omeprazole sodium at 20 mg/kg†
(Mean values with their standard errors for four rats per group)

PPI, proton pump inhibitor.
* Mean value was significantly different from that of the basal value (P < 0·05).
† For details of procedures, see Experimental methods.
Body weight
Body weight at the termination of the experiment was 108·0 (sem 2·9) g in the control/PPI − group, 100·4 (sem 3·0) g in the control/PPI+ group, 107·5 (sem 2·4) g in the DFL/PPI − group and 105·4 (sem 2·8) g in the DFL/PPI+ group, respectively. No significant difference in the final body weight was observed among all groups.
Bone mineral density and bone strength
PPI administration significantly decreased cortical, cancellous and total BMD of the femur in the control group but did not affect these parameters in the DFL group (Table 3). Similar findings were observed in the MMICA and PMICA of the femur. Dry weight, ash weight, Ca content and P content of the femur were also significantly decreased by PPI administration in the control group but were not affected by the administration in the DFL group. Breaking energy of the femur was significantly lower in the control/PPI+ group than in the control/PPI − group but did not differ between the DFL/PPI − and DFL/PPI+ groups.
Table 3 Effects of hypochlorhydria induced by a proton pump inhibitor (PPI) and dietary dairy product fermented by lactobacilli (DFL) on bone parameters of femurs of rats*
(Mean values with their standard errors for eight rats per group)

PPI–, physiological saline injection; PPI+, PPI injection; BMD, bone mineral density; MMICA, minimum moment of inertia of cross-sectional areas; PMICA, polar moment of inertia of cross-sectional areas.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* For details of procedures and diets, see Experimental methods and Table 1.
† Rats given a control diet for 9 d and subcutaneously administered with physiological saline every 12 h from day 5 for 5 d.
‡ Rats given a control diet for 9 d and subcutaneously administered with omeprazole sodium at 20 mg/kg every 12 h from day 5 for 5 d.
§ Rats given a DFL diet for 9 d and subcutaneously administered with physiological saline every 12 h from day 5 for 5 d.
∥ Rats given a DFL diet for 9 d and subcutaneously administered with omeprazole sodium at 20 mg/kg every 12 h from day 5 for 5 d.
Plasma biochemical parameters
Plasma total Ca and P concentrations did not differ among all groups (Table 4). Plasma osteocalcin (a bone turnover marker) and type I collagen C-telopeptides (a bone resorption marker) concentrations were significantly increased by PPI administration in the control group but did not differ between the DFL/PPI − group and the DFL/PPI+ group. There was no significant difference in the plasma 25(OH)D concentration among all groups. Plasma 1,25(OH)2D concentration was significantly increased by PPI administration in the control group but did not differ between the DFL/PPI − group and the DFL/PPI+ group. Plasma intact PTH concentration was significantly increased by PPI administration in the control group but there was no significant difference between the DFL/PPI − group and the DFL/PPI+ group.
Table 4 Effects of hypochlorhydria induced by a proton pump inhibitor (PPI) and dietary dairy product fermented by lactobacilli (DFL) on plasma biochemical parameters of rats*
(Mean values with their standard errors for eight rats per group)

PPI–, physiological saline injection; PPI+, PPI injection; CTX, type I collagen C-telopeptides; PTH, parathyroid hormone; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
a,b Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* For details of procedures and diets, see Experimental methods and Table 1.
† Rats given a control diet for 9 d and subcutaneously administered with physiological saline every 12 h from day 5 for 5 d.
‡ Rats given a control diet for 9 d and subcutaneously administered with omeprazole sodium at 20 mg/kg every 12 h from day 5 for 5 d.
§ Rats given a DFL diet for 9 d and subcutaneously administered with physiological saline every 12 h from day 5 for 5 d.
∥ Rats given a DFL diet for 9 d and subcutaneously administered with omeprazole sodium at 20 mg/kg every 12 h from day 5 for 5 d.
Calcium and phosphorus balance
Food intake during the metabolic experiment did not differ among all groups (Table 5). Ca intake was slightly higher in the control/PPI − group than in the DFL/PPI − group, but did not differ among the other groups. Faecal Ca excretion was significantly increased by PPI administration in the control and DFL groups, and was significantly lower in the DFL/PPI+ group than in the control/PPI+ group. The amount and rate of apparent Ca absorption were significantly decreased by PPI administration in the control and DFL groups, and were significantly higher in the DFL/PPI+ group than in the control/PPI+ group. The amount of urinary Ca excretion was significantly decreased by PPI administration in the control and DFL groups. The amount and rate of Ca retention were significantly decreased by PPI administration in the control and DFL groups, and were significantly higher in the DFL/PPI+ group than in the control/PPI+ group.
Table 5 Effects of hypochlorhydria induced by a proton pump inhibitor (PPI) and dietary dairy product fermented by lactobacilli (DFL) on calcium and phosphorus balance in rats during the metabolic experiment*
(Mean values with their standard errors for eight rats per group)

PPI–, physiological saline injection; PPI+, PPI injection.
a,b,c,d Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
* For details of procedures and diets, see Experimental methods and Table 1.
† Rats given a control diet for 9 d and subcutaneously administered with physiological saline every 12 h from day 5 for 5 d.
‡ Rats given a control diet for 9 d and subcutaneously administered with omeprazole sodium at 20 mg/kg every 12 h from day 5 for 5 d.
§ Rats given a DFL diet for 9 d and subcutaneously administered with physiological saline every 12 h from day 5 for 5 d.
∥ Rats given a DFL diet for 9 d and subcutaneously administered with omeprazole sodium at 20 mg/kg every 12 h from day 5 for 5 d.
P intake did not differ among all groups. No significant difference in the amount of apparent P absorption was observed among all groups. The rate of apparent P absorption was significantly higher in the control/PPI − group than in the DFL groups, but did not differ among the other groups. Urinary P excretion was significantly increased by PPI administration in the control and DFL groups, and was significantly higher in the control/PPI+ group than in the DFL/PPI+ group. The amount of P retention was significantly decreased by PPI administration in the control group but did not differ between the DFL/PPI − group and the DFL/PPI+ group. The rate of P retention was significantly decreased by PPI administration in the control and DFL groups and was significantly higher in the DFL/PPI+ group than in the control/PPI+ group.
Discussion
The purpose of the present study is to investigate whether hypochlorhydria in itself adversely influences bone strength and BMD. We ascertained that the subcutaneous injection with OM sodium at a dose of 20 mg/kg to growing rats moderately increased the pH value of gastric juice to an extent similar to that observed in aged rats (aged 86 weeks)(Reference Uchida, Misaki and Kawano3) and patients with hypochlorhydria(Reference Ayazi, Leers and Oezcelik20). An in vitro study(Reference Tuukkanen and Väänänen21) suggested that PPI might directly influence the proton pump in the osteoclast other than in the stomach, leading to a decrease in bone resorption and an increase in BMD. On the other hand, in the present study, bone resorption was stimulated rather than inhibited by PPI administration in the control group. Although the reason for this discrepancy is unclear, the direct inhibitory effect of PPI on the osteoclastic proton pump may be too small to decrease bone resorption at least in the present study. Therefore, we considered that the pharmacological effect of PPI other than the induction of hypochlorhydria on bone metabolism can be disregarded in the present study.
In the present study, PPI-induced hypochlorhydria decreased femoral strength and BMD as shown by X-ray CT and a practical quantitative method in growing rats fed a control diet. Additionally, we have observed that long-term hypochlorhydria induced by PPI decreased femoral BMD in adult rats (S Takasugi, K Ashida, S Maruyama, Y Komaba, T Kaneko and T Yamaji, unpublished results). These results suggest that hypochlorhydria adversely influences BMD irrespective of age. Kirkpantur et al. (Reference Kirkpantur, Altun and Arici22) reported that maintenance haemodialysis patients taking OM have lower BMD when compared with non-users of acid-suppression drugs, which supports the present results. To clarify the mechanism by which hypochlorhydria induced by PPI decreased BMD, we measured plasma bone turnover marker and bone resorption marker. The present study indicated that hypochlorhydria induced by PPI resulted in high bone turnover, leading to a decrease in BMD and bone strength in the control group.
We also found that hypochlorhydria induced by PPI administration increased plasma PTH concentration in the control group, which probably results in the high bone turnover(Reference Fu, Jilka and Manolagas23). It is well known that PTH plays a predominant role in the maintenance of plasma ionised Ca level, sensing minute-by-minute changes in the plasma ionised Ca(Reference Pérez, Picotto and Carpentieri24). In the present study, PPI-induced hypochlorhydria remarkably increased faecal Ca excretion, and decreased apparent Ca absorption and Ca retention. These results suggest that hypochlorhydria suppressed Ca absorption, subsequently leading to hyperparathyroidism. The decrease in urinary Ca excretion observed in the control/PPI+ group is considered to be due to hyperparathyroidism(Reference Pérez, Picotto and Carpentieri24). We did not observe the decrease in the blood total Ca level in the control/PPI+ group, although plasma PTH level was increased. Tordoff et al. (Reference Tordoff, Hughes and Pilchak25) reported that although short-term Ca deprivation decreased plasma ionised Ca level and increased plasma PTH level, it did not affect plasma total Ca, which is consistent with the present study. PPI-induced hypochlorhydria increased the conversion of 25(OH)D to 1,25(OH)2D in the control group, which probably results from the increase in plasma PTH level(Reference Schiavi and Kumar26). Hypochlorhydria induced by PPI also remarkably increased urinary P excretion, leading to the decrease in P retention in the control group. This increase in the urinary P was likely to be due to the increase in plasma PTH level(Reference Shaikh, Berndt and Kumar27).
We demonstrated that the DFL diet partially improved the effects of PPI-induced hypochlorhydria on apparent Ca absorption and Ca retention. Ca solubility is one plausible mechanism by which hypochlorhydria decreased apparent Ca absorption and the DFL diet improved apparent Ca absorption under hypochlorhydria. The absorbability of Ca is dependent on its solubility. Acidity in the stomach and the resulting chyme increase Ca solubility(Reference Recker16). Therefore, it is likely that PPI-induced hypochlorhydria decreased Ca solubility, leading to the Ca malabsorption, and that the DFL diet improved Ca solubility, leading to a partial improvement of Ca absorption. In fact, we ascertained in an in vitro digestion study with artificial digestive fluid without hydrochloric acid that the soluble Ca fraction of the digest of the DFL diet was about twice higher than one of the control diet even after neutralisation by adding sodium bicarbonate (S Takasugi, K Ashida, S Maruyama, Y Komaba, T Kaneko and T Yamaji, unpublished results). There are two possible explanations why the DFL diet could improve Ca solubility under hypochlorhydria. The first is that the DFL diet contains a high level of l-lactic acid. Tang et al. (Reference Tang, Shah and Wilcox28) reported that Ca-fortified soyamilk fermented with Lactobacillus increased Ca solubility, and the increase is related to a lowered pH associated with l-lactic acid. The second is that the DFL diet contains more soluble Ca sources such as calcium phosphate(Reference Pansu, Duflos and Bellaton29) than calcium carbonate, a practically insoluble Ca source in water(Reference Straub30, Reference Takasugi, Matsui and Yano31). Approximately 100 % of Ca in the control diet is derived from calcium carbonate, while 30 % of Ca in the DFL diet is calcium phosphate derived from the lyophilised DFL and the residual 70 % of Ca is calcium carbonate. On the other hand, we also found that a control diet fortified with as much l-lactic acid as the DFL diet improved apparent Ca absorption to an extent similar to that by the DFL diet in growing rats with hypochlorhydria induced by PPI (S Takasugi, K Ashida, S Maruyama, Y Komaba, T Kaneko and T Yamaji, unpublished results). The control diet fortified with l-lactic acid contains only calcium carbonate as the Ca source. These results support the idea that the DFL diet could improve Ca solubility under hypochlorhydria mainly due to l-lactic acid. However, it remains unclear the extent to which fermentation products such as l-lactic acid in the DFL diet could contribute to the improvement of Ca absorption under hypochlorhydria, and whether unfermented dairy products could also improve Ca absorption under hypochlorhydria, since dairy products including the DFL have some favourable factors for Ca absorption(Reference Tunick32).
The DFL diet cancelled the adverse effects of hypochlorhydria on bone strength, BMD, bone turnover and 1,25(OH)2D, and partially improved the increase in plasma PTH concentration and urinary P excretion, and the decrease in P retention. These results suggest that the ameliorating effects of the DFL diet on Ca absorption under hypochlorhydria resulted in the improvement of plasma PTH and 1,25(OH)2D, leading to the maintenance of normal bone turnover, BMD, bone strength and P metabolism.
Hypochlorhydria is reported to impair the utilisation of other nutrients such as Fe or induce small-intestinal bacterial overgrowth(Reference Pohl, Fox and Fried33). Some researchers have indicated the negative effects of Fe deficiency on bone metabolism in rats(Reference Medeiros, Stoecker and Plattner34) and the adverse effects of small-intestinal bacterial overgrowth on bone(Reference Stotzer, Johansson and Mellström35) and Ca metabolism(Reference Walshe, Healy and Speekenbrink36) in human subjects. In addition, some researchers have suggested the stimulating effect of lactic acid fermentation on Fe absorption in Caco-2 cells(Reference Bergqvist, Andlid and Sandberg37) and the ameliorating effect of yogurt supplementation on small-intestinal bacterial overgrowth in the elderly(Reference Schiffrin, Parlesak and Bode38). It remains uncertain whether the changes in bone and Ca metabolism observed in the present study depend on these previously reported alterations induced by hypochlorhydria. Further study is necessary to clarify these issues.
In conclusion, the present study indicated that PPI-induced hypochlorhydria decreases BMD in growing rats, which is accompanied by high bone turnover induced by secondary hyperparathyroidism due to Ca malabsorption. Additionally, we demonstrated that DFL intake cancels these adverse effects probably via improving Ca malabsorption.
Acknowledgements
The present study received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
S. T. and K. A. designed the research; S. T., K. A., S. M. and Y. K. conducted the research; S. T. analysed the data; S. T., K. A., T. K. and T. Y. wrote the manuscript. S. T. had primary responsibility for the final content. All authors read and approved the final manuscript.
S. T., K. A., S. M., Y. K., T. K. and T. Y. have no conflicts of interest.









