Specific recommendations for intake of vitamin D exist in many countries1–Reference Trichopoulou and Vassilakou3. However, it is not specified in which form vitamin D should preferably be consumed.
There is a clear linear relationship between oral vitamin D intake and the resulting serum 25-hydroxyvitamin D (25(OH)D) concentration, as the hydroxylation of vitamin D is not regulated tightlyReference Holick, Shils, Shike, Ross, Caballero and Cousins4. The serum 25(OH)D response to oral supplementation is positively related to the dose given but inversely related to initial serum 25(OH)D concentrationReference Vieth5, Reference Viljakainen, Palssa, Kärkkäinen, Jakobsen and Lamberg-Allardt6.
Vitamin D deficiency has been proposed to contribute to the development of various diseasesReference Holick7. A Norwegian study found an inverse association between taking cod-liver oil in the first year of life and the risk of type 1 diabetes, but this association was not found for other vitamin D-containing supplementsReference Stene and Joner8. The dose taken was not established, and it is not clear whether the association could be attributed to vitamin D or other components. Other studies have suggested a preventive effect of vitamin D on type 1 diabetes, both epidemiologicalReference Harris9, Reference Hyppönen, Läärä, Reunanen, Järvelin and Virtanen10 and using 1,25-dihydroxyvitamin D in experimental animalsReference Zella, McCary and DeLuca11. However, it is not known whether vitamin D from cod-liver oil and multivitamin supplements is absorbed to the same extent.
There is evidence that biological effects of vitamin A depend on the form in which it is ingestedReference Myhre, Carlsen, Bøhn, Wold, Laake and Blomhoff12, but we are not aware of any data to indicate whether the bioavailability of cholecalciferol differs according to the type of supplement, such as water-miscible tablets or fish oil preparations. Our main objective for the present study was to test whether 4 weeks of daily supplementation with 10 μg cholecalciferol from a fish oil capsule produces a larger increase in serum 25(OH)D concentration compared with the same dose of cholecalciferol given as a multivitamin tablet.
Subjects and methods
Recruitment of subjects and exclusion criteria
Subjects with Norwegian and other backgrounds were recruited principally among medical students and nurse students in Oslo, the intervention starting mid-February 2005. In addition, subjects with Tamil background were recruited through an organisation for Tamils in Oslo (Tamil Resource and Counselling Centre), the intervention starting mid-March 2005. Those who already took a vitamin D supplement regularly, defined as once per week or more, or had been travelling to sunny areas or used a tanning bed during the previous 3 months, were defined as ineligible to participate. A total of seventy-four subjects of the 143 subjects who had agreed to participate (51·7 %) were randomised to receive multivitamin tablets or fish oil capsules (Fig. 1). However, ten individuals did not meet the eligibility criteria (had been travelling to sunny areas or used a tanning bed) on a second inspection, three individuals were non-compliant (defined as taking less than twenty-six tablets during the 28 d period), three individuals were not able to provide the second blood sample, two individuals had their second blood sample drawn 3 d before schedule, and one individual withdrew from the study after randomisation. Exclusion of these subjects was done after randomisation, but before the information about treatment group was unblinded and before performing any statistical analysis. Thus, fifty-five subjects (74·3 % of those randomised) were included in the primary analysis.

Fig. 1 Participant flow.
Randomisation and group allocation
On the day of attendance, those who had agreed to participate and were found eligible signed a written consent form and completed a self-administered questionnaire concerning usual diet and sun exposure. Subsequently, a venous blood sample was drawn, and the participant received a sealed, non-transparent envelope with the allocated intervention. The randomisation procedure was performed beforehand by a statistician by block randomisation with blocks varying in size from four to eight, in order to distribute the participants equally on the two intervention groupsReference Pocock and Pocock13. Group allocation was assigned to each participant according to their number in the sequence of attendance at the first blood sampling. Group allocation was concealed in envelopes numbered in ascending order, which were handed out to the participants in the order that they met. Study personnel involved in recruitment of participants and data collection were blinded to the participants' group allocation.
Intervention
The multivitamin group received a daily supplement of one multivitamin tablet of type Vitaplex ABCD (Cederroth AS, Revetal, Norway), a common vitamin supplement sold in grocery stores in Norway. The fish oil group received a daily supplement of one fish oil capsule, specially manufactured for the present study by Peter Möller (now MöllerCollett AS, Lysaker, Norway). The fish oil capsules were aimed to contain a dose of cholecalciferol identical to the dose already found in the multivitamin tablets by analysis in an independent laboratory (AS Vitas, Oslo, Norway), and similar doses of vitamin A. In addition, the multivitamin tablet contained other water-soluble vitamins, whereas the fish oil capsule contained vitamin E and n-3 fatty acids (Table 1).
Table 1 Nutritional content per tablet of the intervention supplements (Mean values)
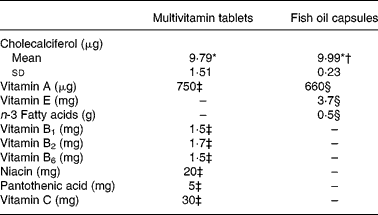
* According to the laboratory analysis at an independent laboratory (AS Vitas, Oslo, Norway), based on analysis of eight fish oil capsules and twenty-four multivitamin tablets.
† Mean vitamin D content of the fish oil capsules when analysed by the manufacturer Peter Möller (now MöllerCollett AS, Lysaker, Norway) was 9·6 μg.
‡ According to the nutritional declaration on the multivitamin tablets.
§ According to the manufacturer Peter Möller (now MöllerCollett AS).
Supplements for 28 d were handed out to each participant at baseline, along with a compliance form. The participants were instructed to mark the intake of a tablet or capsule for each day of the study period, as well as to note any extraordinary event that occurred during the period (for example, forgetting to take a tablet or capsule, or having a vitamin D-rich meal). If forgetting to take a tablet or capsule, the participants were instructed to take two tablets on the following day, in order to take altogether twenty-eight tablets or capsules during the study period. The participants were recommended to take the tablet or capsule with a glass of water. Although meal composition and time since last meal are expected to influence absorption of vitamin D from supplements, subjects were not instructed to standardise meals or time between meals and taking supplements. This was done to minimise interference with daily routines of subjects and thus maximise compliance with taking supplements. Any variation in meal composition and timing should be equally distributed on the two intervention groups. The compliance form was to be returned at the time of the follow-up blood sampling.
Determination of cholecalciferol content of the intervention supplements
The cholecalciferol content of twenty-four randomly selected multivitamin tablets was assessed in an independent laboratory (AS Vitas) by HPLC-UV-MS. After this, but before the start of the intervention, the same analysis was performed in eight fish oil capsules. For the multivitamin tablets, each tablet was crushed in a 10 ml amber vial, and 10 ml 2-propanol–water (75:25, v/v) containing butylated hydroxytoluene as an antioxidant was added. Thorough homogenisation was performed with a hand-held ultraturax motorised homogeniser (Pro 200; Pro Scientific Inc., Oxford, CT, USA), followed by thorough mixing (10 min), ultrasound bath (10 min) and centrifugation (20 min; 2000 g at 10°C). For the fish oil capsules, each capsule was cut open and emptied into a 10 ml amber vial, and 10 ml 2-propanol–water (75:25, v/v) containing butylated hydroxytoluene as an antioxidant was added. The vial containing the extraction liquid, oil and the opened capsule was shaken vigorously for 15 min. For both supplements, 1 ml was transferred to a new vial and a sample of 100 μl was injected into a liquid chromatograph–mass spectrometer (Agilent Technologies, Palo Alta, CA, USA). HPLC was performed with an HP 1100 liquid chromatograph with UV detection and interfaced by atmospheric pressure electrospray ionisation to an HP mass spectrometric detector. Cholecalciferol and ergocalciferol were separated on a 4·6 mm × 150 mm reversed phase C8 column (Agilent Eclipse XDB-C8 4·6 × 150 mm, 5 μm; Agilent, Palo Alto, CA, USA). Elution was performed with mobile phase A (0·5 % ammonium acetate) and B (methanol 0·5 % ammonium acetate). The gradient used was 80 % B at 0 min to 100 % B at 6 min. The column temperature was 40°C. A one-point calibration curve was made from analysis of ethanol solution enriched with known cholecalciferol concentration. Recovery was > 99 %. The method is linear from 1–30 μg/ml and the limit of detection was 0·1 μg/ml. The CV for the method as stated by the laboratory was 0·7 %.
The mean content of cholecalciferol was 9·79 (sd 1·51) μg per multivitamin tablet, and 9·99 (sd 0·23) μg per fish oil capsule.
Sample size
We considered it relevant to detect a mean difference in increase in serum 25(OH)D between the two groups of 10–15 nmol/l. If one supplement led to a mean increase in serum 25(OH)D by 31·3 nmol/l and the other supplement led to a mean increase in serum 25(OH)D by 20 nmol/l, the standardised effect size would be 0·75 sd (mean difference in increase of 11·25 nmol/l), giving a power of 80 % to detect a significant difference between the two types of supplements in a study with twenty-eight subjects in each of two equally sized groups. Our final sample of those who completed the study and satisfied the eligibility criteria consisted of fifty-five individuals, n 28 in the multivitamin group and n 27 in the fish oil capsule group.
Data collected at baseline
Each participant completed a self-administered three-page questionnaire before allocation. During completion of the questionnaire, they had the possibility to ask for assistance (i.e. clarification of questions, or language issues) from one of the project leaders. The questionnaire included questions about usual intake of vitamin D-containing foods, supplement use, clothing and sun-exposure habits, as well as self-reported height and weight, date of birth, education and ethnic background. At the follow-up meeting, all participants' height and weight were measured with the same electronic height- and weight-measuring device.
Collection and analysis of blood samples
Blood samples were centrifuged (10 min; 2000 g at 10°C) within 30 min after blood collection and were immediately frozen. Serum samples were kept frozen at − 70°C until analysed. Serum 25(OH)D was measured by RIA (DiaSorin Inc., Stillwater, MN, USA) in the Hormone Laboratory, Aker University Hospital (Oslo, Norway). This assay measures both 25(OH)D3 and 25-hydroxyvitamin D2. The intra- and inter-assay CV were 6 and 14–15 %, respectively. The detection limit was 6 nmol/l.
Statistical analysis
Statistical analysis was performed with SPSS version 14.0 for Windows (SPSS Inc., Chicago, IL, USA). For the a priori defined principal analysis, we entered increase in serum 25(OH)D concentration as the dependent variable in a linear regression analysis with intervention group as the exposure variable with adjustment for baseline serum 25(OH)D concentrationReference Vickers and Altman14. We chose a significance level of 0·05. We also performed additional analyses that included BMI and ethnic background as independent variables. When performing additional analyses we aimed for the model that would optimally correct for confounding and maximise the precision of the effect estimate of intervention group.
Results
Baseline characteristics
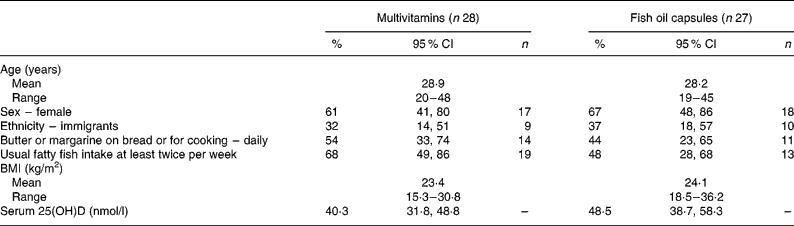
In total, fifty-five individuals, twenty-eight in the multivitamin group and twenty-seven in the fish oil group, fulfilled the eligibility criteria and completed the study (Fig. 1). Except for a difference in serum 25(OH)D concentration of 8·2 (95 % CI − 4·4, 20·9) nmol/l, there were no striking differences in baseline characteristics between the two groups (Table 2). Mean baseline serum 25(OH)D concentration was 44·3 (sd 23·6) nmol/l (n 55). At baseline, 60 % of the subjects had serum 25(OH)D concentration < 50 nmol/l, 24 % had serum 25(OH)D concentration < 25 nmol/l, and one individual had serum 25(OH)D concentration < 12·5 nmol/l.
Table 2 Baseline characteristics of subjects according to type of vitamin D supplementation (Percentages and 95 % confidence intervals)
Effect of intervention
During the intervention period, the mean increase in serum 25(OH)D concentration was 36 (95 % CI 32, 40) nmol/l in the multivitamin tablet group and 32 (95 % CI 26, 38) nmol/l in the fish oil capsule group. When controlling for baseline serum 25(OH)D concentration, the mean difference in the increase in serum 25(OH)D between the intervention groups was 3·5 (95 % CI − 3·6, 10·6) nmol/l (P = 0·33) (Fig. 2). Adjustment for BMI and ethnic background yielded a similar estimate (2·8 (–4·7, 10·2) nmol/l; P = 0·46). Adjustment for age and sex did not alter the estimate. When we also included the ten subjects who were excluded from the primary analysis because they had travelled to sunny areas or taken vitamin D supplements before study start (total n 65), the mean difference in increase after supplementation with fish oil and multivitamins was 3·6 (95 % CI − 3·4, 10·5) nmol/l. When we further included the three who did not comply with supplementation and the two who had their second blood sample drawn 3 d ahead of schedule (total n 70), the mean difference in increase after supplementation with fish oil and multivitamins was 2·8 (95 % CI − 3·8, 9·3) nmol/l.

Fig. 2 Individual serum 25-hydroxyvitamin D concentrations at baseline and after intervention according to type of cholecalciferol supplement: multivitamin tablets (●; n 28) or fish oil capsules (Δ; n 27). (----), Mean linear increase, multivitamin tablets; (—), mean linear increase, fish oil capsules.
After 4 weeks of supplementation, overall mean serum 25(OH)D concentration was 78·4 (sd 24·5) nmol/l (n 55), and it did not differ between the intervention groups (P = 0·56). A total of 9 % (five subjects) now had serum 25(OH)D concentration < 50 nmol/l, and none had serum 25(OH)D < 25 nmol/l.
Increase in serum 25-hydroxyvitamin D according to ethnic background, sex or baseline serum 25-hydroxyvitamin D concentration
When pooling the data of the two intervention groups (n 55), increase in serum 25(OH)D did not vary with respect to ethnic background (P = 0·88) or sex (P = 0·69). In a linear regression model, change in serum 25(OH)D did not depend significantly on baseline serum 25(OH)D (P = 0·13 in bivariate analysis). However, when excluding two outliers who had baseline concentration above 100 nmol/l, there was a significant inverse linear relationship between baseline serum 25(OH)D and change in serum 25(OH)D (P = 0·002).
Discussion
To the best of our knowledge, this is the first randomised trial comparing the increase in serum 25(OH)D in groups receiving the same oral dose of vitamin D in two different supplemental forms, and we found a similar mean increase in serum 25(OH)D concentration after 4 weeks' intake of multivitamin tablets or fish oil capsules both containing 10 μg cholecalciferol per d. The increase in serum 25(OH)D concentration was not related to sex, ethnic background or BMI. The present study also showed that vitamin D deficiency was prevalent in February and March among healthy, young subjects who lived at 60°N and who had not been travelling to sunny areas or taken vitamin D supplements during the winter. However, 4 weeks of supplementation with 10 μg cholecalciferol per d increased mean serum 25(OH)D to an adequate level, i.e. from 44 to 78 nmol/l, during the 4-week period. Similar results were found in a small supplementation study in young healthy students in Northern IrelandReference Barnes, Robson, Bonham, Strain and Wallace15, who had a mean increase from 48 to 87 nmol/l when receiving a daily dose of 15 μg for 8 weeks, and in a study of out-patients with mean age 53 years in CanadaReference Vieth, Kimball, Hu and Walfish16 who had a mean increase from 48 to 79 nmol/l when receiving a weekly dose of 95 μg for more than 6 months. A summary of a large amount of supplementation studies published up to 1999 showed that a daily dose of 10 μg vitamin D gave an average increase in 25(OH)D of 31 nmol/l, although with large variation between studies, indicating that additional factors may influence the degree of increase.Reference Vieth5 Only one of the summarised studies had been performed in young adults, with mean age 21 yearsReference Davie, Lawson, Emberson, Barnes, Roberts and Barnes17. In that study, nine individuals received 10 μg daily for a period of 2·3 months, and the mean increase in serum 25(OH)D was 41·5 nmol/l.
Increase in serum 25(OH)D after supplementation is known to be inversely related to baseline 25(OH)D concentrationReference Lips, Duong, Oleksik, Black, Cummings, Cox and Nickelsen18. There was a large variation in baseline serum 25(OH)D in our data (range 9–104, sd 23·6 nmol/l), perhaps explaining why the significant inverse linear relationship between baseline concentration and increase in serum 25(OH)D was only evident after exclusion of two outliers. Although several studies exist on the effect of vitamin D supplementation on serum 25(OH)D concentration, we are not aware of any other studies that have compared the effect on 25(OH)D concentration of two different forms of cholecalciferol supplements. However, a Finnish study group evaluated the bioavailability of cholecalciferol from fortified bread, and found that 10 μg cholecalciferol from bread increased serum 25(OH)D concentration as efficiently as 10 μg cholecalciferol from a supplement during a 3-week periodReference Natri, Salo, Vikstedt, Palssa, Huttunen, Karkkainen, Salovaara, Piironen, Jakobsen and Lamberg-Allardt19.
The study had sufficient power to detect a mean difference in increase of serum 25(OH)D of 10–15 nmol/l or more, which we considered clinically relevant. However, we cannot exclude the possibility that more subtle differences exist.
We excluded a few subjects from the principal analysis because they reported taking less than twenty-six capsules or tablets during the study period or because they after randomisation were found to have violated the eligibility criteria. However, the dropout was similarly distributed on the two intervention groups and the decision to exclude these was made before unblinding group allocation and before data analysis. Furthermore, performing intention-to-treat analysis including all randomised subjects who had both blood samples drawn in the statistical analysis, regardless of whether they completed the study did not alter the results, supporting the principal analysis.
We conclude that fish oil capsules and multivitamin tablets containing 10 μg cholecalciferol produced a similar mean increase in serum 25(OH)D concentration over a 4-week period.
Acknowledgements
Specially manufactured fish oil capsules were provided by Peter Möller (now MöllerCollett AS). The blood sample analyses were funded by Aktieselskabet Freia Chocolade Fabriks Medisinske fond. K. H.’s doctoral grant was financed with the aid of EXTRA funds from the Norwegian Foundation for Health and Rehabilitation. We are grateful to those who participated in the study, as well as bioengineer Eva Kristensen who drew the blood samples. The study protocol was reviewed by the Regional Committee for Medical Research Ethics and approved by the Norwegian Data Inspectorate. Written informed consent was collected from all participants.








