Introduction
The explosive growth of pine plantation silviculture in the south-eastern United States during the past century has raised concerns among conservationists who have warned that short harvesting rotations geared toward maximum production of wood products will lead to a decrease in avian species diversity and abundance. This alarm was summarized by Terborgh (1989:168) “These southern pine plantations are a biological desert. One finds a few pine warblers, cardinals, and towhees, but little else….Although classified as ‘forest’ in land-use surveys, pine plantations are hardly better as habitat for migrants and other wildlife than… Iowa cornfields.”
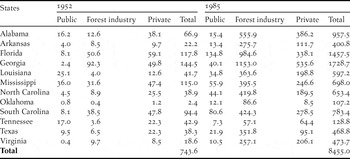
Until the early 20th century, southern forest lands were left to regenerate naturally after cutting and millions of hectares of cutover lands lay in waste (Wakeley Reference Wakeley1945, Williams Reference Williams1989). Less than 200 ha of pine plantations had been planted by 1920 in the vast pine belt extending from eastern Texas to Virginia (Wakeley Reference Wakeley1954). After the first experimental pine plantations were established in Louisiana in 1922, plantation silviculture developed rapidly in the 1930s, was slowed during World War II, and then increased swiftly in the post-war era (Wakeley Reference Wakeley1954). The area successfully planted with pine increased to 0.30 million ha by 1945 (Wakeley Reference Wakeley1945). By 1952, pine plantations were planted on 0.74 million ha in 12 south-eastern states (USDA Forest Service 1988). By 1985, this total had increased more than tenfold to 8.46 million ha, mostly on forest industry and private lands (Table 1). By 2010, more than 15.8 million ha were managed as pine plantations (Wear and Greis Reference Wear and Greis2012).
Table 1. Area (in thousands of hectares) of pine plantations in the south-eastern United States in 1952 and 1985 (extracted from USDA Forest Service 1988), the last year for which plantation statistics by state and ownership type were available before the publication of Terborgh’s Reference Terborgh1989 essay.
What is not generally recognised in ecological literature is that the typical industrial pine plantation, composed of densely planted even-age stands covering 5–200 ha, represents a fundamentally new wildlife habitat in the south-eastern United States. At the time of Terborgh’s essay (1989), southern pine silviculture was little more than 60 years old (Wakeley Reference Wakeley1954, Fox et al. Reference Fox, Jokela and Allen2007) and few studies of avian communities in southern pine plantations had been published (Dickson and Segelquist Reference Dickson and Segelquist1979, Repenning and Labisky Reference Repenning and Labisky1985). Although the deleterious effects of short-rotation plantation forestry were known for the Red-cockaded Woodpecker Picoides borealis, the only avian species in the south-eastern United States that requires old growth pines (Ligon Reference Ligon1970, Jackson et al. Reference Jackson, Lennartz and Hooper1979), there were limited published data to indicate that plantation silviculture might have neutral or even positive effects on populations of some migratory and resident avian species. It is now known that a number of species attain high breeding populations in southern pine plantations (Dickson and Segelquist Reference Dickson and Segelquist1979, Dickson et al. Reference Dickson, Conner and Williamson1993, Wilson and Watts Reference Wilson and Watts2000, Henry Reference Henry2004, Loehle et al. Reference Loehle, Wigley, Rutzmoser, Gerwin, Keyser, Lancia, Reynolds, Thill, Weih, White and Wood2005, Bassett-Touchell and Stouffer Reference Bassett-Touchell and Stouffer2006, Hazler et al. Reference Hazler, Amacher, Lancia and Gerwin2006, Legrand et al. Reference Legrand, Chamberlain and Moser2007, Hanberry et al. Reference Hanberry, Hanberry, Riffell, Demarais and Jones2012).
In this paper, I report the large-scale colonisation of southern pine plantations by Swainson’s Warbler Limnothlypis swainsonii, a rare migratory songbird which ranks near the top of state and federal species lists of conservation concern in the United States (Hunter et al. Reference Hunter, Pashley and Escano1993). Low population size has been attributed to habitat limitation on the breeding grounds (Graves Reference Graves2001, Reference Graves2002) and a small wintering range in the Caribbean basin (Terborgh Reference Terborgh1989). A recent analysis of North American Breeding Bird Survey (BBS) data estimated the global breeding population at 90,000 individuals, sparsely distributed across 15 states from Texas to Virginia (Partners in Flight Science Committee 2013). The endangered Kirtland’s Warbler Setophaga kirtlandii is the only Nearctic-Neotropical migratory songbird breeding in eastern North America with a lower global population (Partners in Flight Science Committee 2013). Partners in Flight assigns a Continental Concern Score (CCS), ranging from 4 (lowest concern) to 20 (highest concern), to avian species that breed in the United States (Partners in Flight Science Committee 2012). Swainson’s Warbler received a CCS of 13. Had it not been for a recent uptrend in BBS population data, which will be discussed later in this paper, it would likely have been assigned a CCS of 16–17 (high conservation concern) based on its historic rarity.
Interest in the natural history of Swainson’s Warbler has been unusually intense since it was discovered in the early 1830s in South Carolina (Audubon Reference Audubon1834, Brewster Reference Brewster1885, Meanley Reference Meanley1971, Graves et al. Reference Graves, Simpson and Stephens1996). Knowledge of its breeding habitat has grown incrementally since the 1880s when a breeding population was discovered in tangled thickets and canebrakes on the coastal plain of South Carolina (Brewster Reference Brewster1885). Until the 1940s, the warbler was widely considered a habitat specialist restricted to canebrakes in lowland swamps and bottomland forest. The known diversity of breeding habitats took a quantum leap when this secretive warbler was discovered in rhododendron and mountain laurel thickets in the cooler altitudes of the Appalachian Mountains (Brooks and Legg Reference Brooks and Legg1942). This finding challenged the prevailing wisdom on habitat stereotypy and provoked disbelief in more than a few sceptics until specimens and evidence of nesting were procured (Murray Reference Murray1939, Brooks and Legg Reference Brooks and Legg1942). One prominent ornithologist commented: “No more amazing example of distribution could be imagined than the occurrence of this bird of the southern swamps in such places” (Murray Reference Murray1939). In hindsight, the discovery of breeding warblers in habitats as varied as rhododendron thickets in the Appalachian Mountains and lowland canebrakes hinted at a latent plasticity in habitat selection. By the 1990s, breeding had been documented in a surprisingly wide spectrum of habitats including disturbance gaps in old growth bottomland forest, regenerating broadleaf clearcuts, pocosins and bays, riverside canebrakes, palmettos in bottomland forest, and Appalachian thickets (Meanley Reference Meanley1971, Eddleman et al. Reference Eddleman, Evans and Elder1980, Graves Reference Graves2001, Reference Graves2002).
A second remarkable expansion in habitat selection was reported in 1992 when Swainson’s Warbler was found nesting in young loblolly pine plantations in eastern Texas (Carrie Reference Carrie1996). Other published reports of breeding in unthinned pine plantations were forthcoming from south-eastern Louisiana (Henry Reference Henry2004, Bassett-Touchell and Stouffer Reference Bassett-Touchell and Stouffer2006, Legrand et al. Reference Legrand, Chamberlain and Moser2007). As unanticipated as these reports were, they only hint at the true geographic extent of pine plantation occupancy on the coastal plain of the south-eastern United States. Here I report dozens of breeding populations of Swainson’s Warbler in pine plantations in 10 states from Texas to Virginia discovered during two decades of field surveys (Figure 1). I provide information on (1) the stand height and age of occupied pine plantations, (2) co-factors such as soil type associated with occupancy, (3) decadal chronology of plantation colonisation, (4) evidence of reproduction in pine plantations, and (5) the geographic extent of colonization of pine plantations. Finally, I discuss the influence of pine silviculture on the conservation of Swainson’s Warbler.
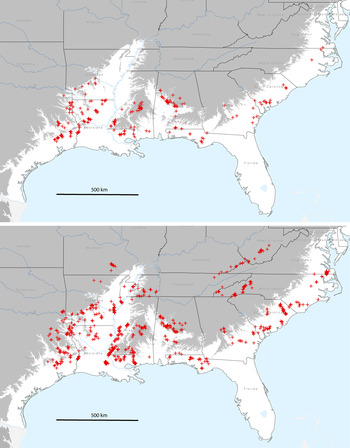
Figure 1. Geographic distribution of Swainson’s Warbler breeding territories in pine plantations (top panel; n = 297) and in habitats other than pine plantations (lower panel; n = 1,402). Grey shading indicates area above 100 m (asl).
Methods
Survey of breeding populations
Geographic surveys of Swainson’s Warbler breeding populations were conducted from 1988 through 2014 (Graves Reference Graves1992, Reference Graves1998, Winker et al. Reference Winker, Glenn and Graves1999, Reference Winker, Graves and Braun2000, Graves Reference Graves2001, Reference Graves2002, Winker and Graves Reference Winker and Graves2008). Some territories were located along established census transects (Graves Reference Graves2001, Reference Graves2002) but most were detected during the systematic exploration of habitat along secondary and primitive roads and less frequently on foot trails or by canoe. Most territories were located with the aid of playback of recorded songs. The majority of males in coastal plain populations arrive on breeding territories between 10 April and 15 April (Meanley Reference Meanley1971). Females arrive about a week later. Egg dates in Louisiana have been recorded as early as 19 April (Henry Reference Henry2004). Strong response to playback, the reluctance to stray from a particular area during “playback-and-follow” trials (Graves Reference Graves1996), mate guarding, and counter-singing with other males were interpreted as evidence of male territorial behaviour. Because of large territory size (Graves Reference Graves2001, Anich et al. Reference Anich, Benson and Bednarz2009), I conservatively considered any subsequent response within 200 m of the original discovery point to represent the same individual unless two or more males were heard singing simultaneously. Territorial determinations were made from 22 April to 30 June.
Classification of habitat and measurement of stand height
A forest stand was classified as a pine plantation if 50% or more of the stand was composed of native pines (loblolly Pinus taeda; slash, P. elliotii; shortleaf, P. echinata; longleaf, P. palustris) established by planting or direct seeding (USDA Forest Service 1988). Evidence of row spacing was obvious in most commercial pine plantations. A few plantations contained a mixture of loblolly and slash or longleaf pines. However, most plantations surveyed in the breeding range of Swainson’s Warbler were planted exclusively in loblolly pine. Many warbler territories in pine plantations also encompassed narrow buffer strips of broadleaf streamside or roadside vegetation. If most of the territory overlapped stands of planted pines, then the territory was classified as “pine plantation.” In the remainder of the paper, I refer to pine plantations that held warbler territories as “occupied.” However, the failure to detect a territorial male did not necessarily mean that a pine plantation was unoccupied because males may have been beyond the effective range of playback during the survey. Thus, I was unable to estimate the proportion of pine plantations that supported breeding territories. It is important to stress that this was not a study of habitat selection in which census sites were randomly chosen and then checked for territorial occupancy.
Several definitions of even-age stand height in pine plantations have been proposed for site indexing (Sharma et al. Reference Sharma, Amateis and Burkhart2002). I defined stand height as the midpoint between the tallest tree (m) in the stand and the average height of the subdominants. Tree heights were determined with optical and laser rangefinders. Owing to time constraints, I made no attempt to quantify plantation size, habitat fragmentation, or the differences between occupied and unoccupied pine plantations. Nor did I attempt to directly age pine plantations, measure spacing or thinning, or ascertain pre-planting treatments or herbicide use in stands.
Characterisation of soils
Swainson’s Warbler is a terrestrial dead-leaf specialist (Meanley Reference Meanley1970, Graves, Reference Graves1998) that feeds on litter arthropods (Savage et al. Reference Savage, Moorman, Gerwin and Sorenson2010, Brown et al. Reference Brown, Benson and Bednarz2011). The distribution of soils is believed to be an important determinant of local and regional patterns of habitat selection in Swainson’s Warbler (Graves Reference Graves1998, Reference Graves2001, Reference Graves2002), to the extent that soil type and moisture influence the accumulation and decay of leaf litter and the taxonomic diversity and abundance of litter arthropods.
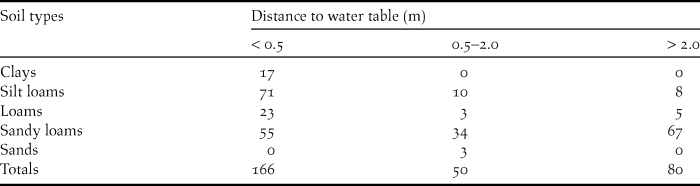
I compiled soil data for Swainson’s Warbler territories located in pine plantations from the online database maintained by the United States Department of Agriculture (Natural Resources Conservation Service 2013). Soil type and distance to water table, two pedological characteristics that correlate with soil moisture, were recorded for each territory. Soil types form a continuum that varies in accordance with a range of factors, including the frequency of soil particle sizes, horizon depths, depositional history, drainage, and slope. I binned soil types in five general categories ranked by the predominant particle size, from fine to coarse: (1) clays; (2) silt loams, (3) loams, (4) sandy loams, and (5) sands. The distance to water table for each soil type was generally presented as a range (in inches) in the online database. For purposes of classification, the minimum distance-to-water table (DWT) value for each soil type was binned in three distance categories: (1) < 0.5 m; (2) 0.5–2.0 m; and (3) > 2.0 m.
Results
Swainson’s Warblers (n = 297 territories) were detected in pine plantations across a wide swath of the coastal plain from eastern Texas to south-eastern Virginia from 1995 to 2014 (Figure 1). As a measure of geographic dispersion, territories in pine plantations were located in 95 counties or parishes in 10 states. An additional 1,402 breeding territories were located in other habitats from 1988 to 2014 (Fig. 1).
Pine plantations were planted on a wide variety of soil types. Most plantations occupied by warblers were planted on sandy loams, with fewer on loams, silt loams and clays (Table 2). A majority of territories were on soils that normally have high water tables (< 0.5 m below the surface). Most territories were underlain by one soil type (n = 187), with fewer on two (n = 108), or three soils (n = 2). Territories overlapping two or more soil types were usually located at the ecotone between floodplains and better drained flanking slopes. The elevation of occupied pine plantations varied from 2–182 m (above sea level): 0–50 m (n = 132 territories); 51–100 m (n = 117 territories); 101–150 m (n = 44 territories); >150 m (n = 4 territories). Highland sites (> 100 m; 16.2 % of total) occurred primarily in the loess bluffs from the Tunica hills of southern Louisiana through central Mississippi and in several locations above the fall line in Alabama.
Table 2. The number of Swainson’s Warbler territories in pine plantations by soil type and distance to water table (DWT). For territories overlying two or more soil types, data are presented for the soil with the minimum DWT.
Warbler territories in pine plantations occurred in a relatively narrow range of stand heights, mostly 6–12 m (Figure 2). No territories were observed in stands less than 4 m tall and few were located in plantations taller than 14 m. The median height of pines in occupied plantations was 7.5 m (
![]() $$\bar x$$
= 8.4 ± 2.3; range, 4–22.5 m, n = 256) (Figures 2 and 3). Most occupied plantations had a broadleaf component (saplings, vines, shrubs), particularly at roadsides and along stream margins.
$$\bar x$$
= 8.4 ± 2.3; range, 4–22.5 m, n = 256) (Figures 2 and 3). Most occupied plantations had a broadleaf component (saplings, vines, shrubs), particularly at roadsides and along stream margins.
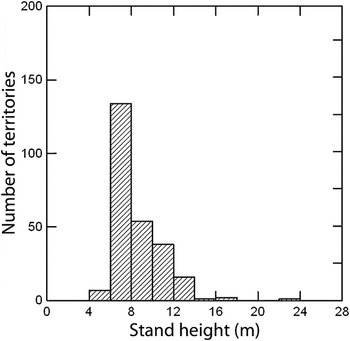
Figure 2. Stand height (m) of pine plantations occupied by breeding Swainson’s Warbler (n = 256 territories).

Figure 3. Young loblolly pine plantations occupied by territorial male Swainson’s Warblers in Escambia County, Alabama (top panel, stand height = 9.5 m) and in Neshoba County, Mississippi (bottom panel, stand height = 6.5 m).
Discussion
Short-rotation pine plantations (25–35 year cycles) represent a fundamentally new ecosystem in the south-eastern United States that has increased in area by five orders of magnitude in the past century from less than 200 ha in 1920 to more than 15.8 million ha today (Wakeley Reference Wakeley1945, Reference Wakeley1954, Wear and Greis, Reference Wear and Greis2012). Before the 1920s, large contiguous tracts of densely-planted, even-aged monocultures of pine were non-existent. In essence, southern pine silviculture constitutes one of the largest habitat manipulation experiments conducted in North America since the arrival of Europeans, yet our understanding of the effects of plantation silviculture on avian populations and the degree to which migratory and resident species have adapted to the novel physiognomy of early seral stages of even-aged pine plantations is rudimentary at best. This stems from the fact that there have been no comprehensive longitudinal studies of avian community composition in pine plantations from planting to harvest, with the possible exception of Dickson et al. (Reference Dickson, Conner and Williamson1993). To compound matters, none of the previous studies have focused on the distribution and abundance of focal species in southern pine plantations at multiple sites across the vast southern pine belt. The present survey of Swainson’s Warbler breeding populations offers new insight on the nature and geographic extent of pine plantation colonisation.
Age and stand height of pine plantations occupied by Swainson’s Warbler
Growth rates of southern pines depend on site characteristics, planting density, thinning, fertilization, and volume of broadleaf vegetation (Wakeley Reference Wakeley1954). On average, the height of dominant trees in loblolly pine stands on coastal plain sites reaches 4 m about six years after planting, 8 m after 10 years, and 12 m after 15 years (Amateis and Burkhart Reference Amateis and Burkhart1985). My survey data show that > 90% of warbler territories occurred in stands 6–12 m tall (corresponding to 8–15 years after planting)(Figure 3). This suggests that unthinned even-aged pine plantations provide suitable breeding habitat for ∼7–8 years during the typical 25–35 year rotation cycle, providing that other requirements are met such as planting density, soil type, distance from water table, and interspersed broadleaf saplings, shrubs, and vines. If silvicultural practices shift toward wider tree spacing, earlier thinning, or a greater application of herbicides to reduce broadleaf growth, then suitability for Swainson’s Warbler will commensurately decrease.
Earlier reports of Swainson’s Warbler in pines gave similar ranges of plantation ages. Carrie (Reference Carrie1996) reported that occupied loblolly pine plantations in eastern Texas ranged in age from 3 to 18 years (
![]() $$\bar x$$
= 14.0 ± 4.2 years, n = 11). In south-eastern Louisiana, the warbler was found breeding in unthinned loblolly pine stands that were 9–14 years old (Henry Reference Henry2004) and in 7–24 year old stands (Bassett-Touchell and Stouffer Reference Bassett-Touchell and Stouffer2006). A third study in the same area of Louisiana found Swainson’s Warblers in unthinned pine stands that were 7–9 years old (Legrand et al.
Reference Legrand, Chamberlain and Moser2007). In contrast, occupancy of broadleaf habitats is largely uncoupled from canopy height and tree age as long as the understorey stem density is sufficient (Graves Reference Graves2001, Reference Graves2002).
$$\bar x$$
= 14.0 ± 4.2 years, n = 11). In south-eastern Louisiana, the warbler was found breeding in unthinned loblolly pine stands that were 9–14 years old (Henry Reference Henry2004) and in 7–24 year old stands (Bassett-Touchell and Stouffer Reference Bassett-Touchell and Stouffer2006). A third study in the same area of Louisiana found Swainson’s Warblers in unthinned pine stands that were 7–9 years old (Legrand et al.
Reference Legrand, Chamberlain and Moser2007). In contrast, occupancy of broadleaf habitats is largely uncoupled from canopy height and tree age as long as the understorey stem density is sufficient (Graves Reference Graves2001, Reference Graves2002).
Co-factors in the occupancy of pine plantations
The same factors that appear to influence breeding habitat selection of Swainson’s Warbler in broadleaf bottomland forests (Meanley Reference Meanley1971, Eddleman et al. Reference Eddleman, Evans and Elder1980, Graves Reference Graves2001, Reference Graves2002, Peters et al. Reference Peters, Lancia and Gerwin2005, Brown et al. Reference Brown, Benson and Bednarz2009) appear to be operative in pine plantations. The common denominator linking breeding territories in pine plantations, bottomland broadleaf forests, and Appalachian forests is high stem or foliar density in the understory (Graves Reference Graves2001, Reference Graves2002, Henry Reference Henry2004, Bassett-Touchell and Stouffer Reference Bassett-Touchell and Stouffer2006). Although the growth form of even-aged pine plantations differs from that of dense stands of broadleaf saplings and shrubs, the foliar distribution and visual screening effects are similar. Local breeding populations of Swainson’s Warbler abandon both pine plantations and broadleaf habitats when the understorey thins due to stand maturation and canopy closure.
Most occupied pine plantations exhibit a significant broadleaf component along roadsides and stand borders (Carrie Reference Carrie1996, Henry Reference Henry2004, Bassett-Touchell and Stouffer Reference Bassett-Touchell and Stouffer2006). As previously mentioned, the warbler forages almost exclusively in terrestrial dead-leaf litter (Meanley Reference Meanley1970, Graves Reference Graves1998). Although their foraging behaviour in pine plantations is unknown, published data from broadleaf habitats (Graves Reference Graves1998, Brown et al. Reference Brown, Benson and Bednarz2011) suggest that deciduous leaf litter is an integral component of breeding territories. It remains to be determined whether this is true for territories in pine plantations.
Occupied pine plantations were planted on a variety of soil types (Table 2). A surprisingly large number of territories occurred on sandy loams with comparatively low water tables (distances > 2 m). This observation suggests four possibilities:
-
Rainfall events are frequent enough to maintain adequate moisture in the leaf litter on well drained soils.
-
Soil moisture may not be as critical to the litter arthropods consumed by Swainson’s Warbler as is generally believed.
-
Dense shade and thermal insulation of young pine plantations facilitates the retention of residual moisture and the maintenance of elevated humidity levels in the leaf litter during rainless periods.
-
Major soil types encompassed local areas of moister soil that were too small to map.
Data in hand are insufficient to determine which explanation is correct. In a broader context, pine plantation silviculture appears to facilitate the warbler’s upslope colonisation of drier sandy loams.
The chronology of colonisation of pine plantations
The chronology of colonisation is poorly known because few biologists systematically searched for Swainson’s Warbler in pine plantations before Carrie’s report (1996) in San Jacinto County, Texas. Other studies of unthinned pine plantations in eastern Texas conducted from 1975 through 1992 found no evidence of breeding warblers. Dickson and Segelquist (Reference Dickson and Segelquist1979) failed to find the species in even-aged pine stands (mean stand height, 4.4 and 13.5 m) in Nacogdoches County, Texas in 1975. In the most comprehensive study, annual censuses in a large pine plantation (∼5 km2) in the same county from 1977 (two years after planting) through 1992 (17 years after planting) also failed to detect Swainson’s Warbler (Dickson et al. Reference Dickson, Conner and Williamson1993). A third study of breeding bird populations in Cherokee, Houston, and Nacogdoches counties in eastern Texas that focused on narrow broadleaf streamside corridors in young pine plantations in 1984–1985 reported similar negative results (Dickson et al. Reference Dickson, Williamson, Conner and Ortego1995). My surveys in eastern Texas did not commence until 2008, so they provide little information on the timing of colonisation. However, Swainson’s Warbler now occurs in pine plantations in Cherokee, Houston, and Nacogdoches counties as well the remainder of breeding range in eastern Texas, with documented territories in 17 counties (Figure 1).
Colonisation of pine plantations in other parts of the warbler’s breeding range is likely to have occurred at least one to two decades before it was actually documented. In 1974, Swainson’s Warbler was reported breeding in control plots of mature mixed broadleaf-pine forest in Livingston Parish, Louisiana, but not in loblolly pine plantations, six, 20, and 46 years old (Noble and Hamilton Reference Noble and Hamilton1975). In 1998–1999, the warbler was reported breeding in loblolly pine plantations in adjacent Tangipahoa Parish, and in 2000 from Livingston Parish and three additional parishes in south-eastern Louisiana (Bassett-Touchell and Stouffer Reference Bassett-Touchell and Stouffer2006). Overall, they detected Swainson’s Warblers in 23 out of 124 pine plantations (Bassett-Touchell and Stouffer Reference Bassett-Touchell and Stouffer2006). Henry (Reference Henry2004) and Legrand et al. (Reference Legrand, Chamberlain and Moser2007) found the warbler to be a predictable breeder in pine plantations in the same area during 2002–2004. My recent surveys (2007–2011) documented breeding territories in pine plantations in 21 parishes in Louisiana distributed throughout the pine-dominated regions of the state (Figure 1).
The unfolding story is similar in the remainder of the warbler’s breeding range on the coastal plain. Recent surveys (2006–2014) documented the occupancy of unthinned pine plantations in Alabama (n = 14 counties), Mississippi (n = 14 counties), Arkansas (n = 8 counties), Georgia (n = 7 counties), South Carolina (n = 6 counties), Florida (n = 5 counties), North Carolina (n = 2 counties), and Virginia (n = 1 county). Collectively, these data suggest that range-wide colonisation of pine plantations occurred after the 1970s, catalysed by the massive post-World War II increase in plantation silviculture.
Population trends from the North America Breeding Bird Survey (BBS) from eastern Texas are consistent with the hypothesis that widespread colonisation of pine plantations occurred in the early 1990s. The BBS is a cooperative census programme conducted annually since 1966, involving thousands of census routes and observers in the United States and Canada (Sauer et al.
Reference Sauer, Hines, Fallon, Pardieck, Ziolkowski and Link2012). Each route has 50 census stops. BBS data were downloaded from the website maintained by the U.S. Geological Survey (Patuxent Wildlife Research Center 2013). Analysis was restricted to census routes in eastern Texas because the most comprehensive early studies of avian populations in young pine plantations (1975–1992) were conducted in this area (Dickson and Segelquist Reference Dickson and Segelquist1979, Dickson et al.
Reference Dickson, Conner and Williamson1993, Dickson et al.
Reference Dickson, Williamson, Conner and Ortego1995) and because Texas supports 33% of the global breeding population of Swainson’s Warbler (Partners in Flight Science Committee 2013). Comparison was restricted to routes (n = 20) on which Swainson’s Warbler had been recorded at least once during the review period (1980–2012). The number of routes censused in a given year varied from six to 18 (
![]() $$\bar x$$
= 11.5 ± 4.5). BBS data show a marked non-linear increase from 1980 to 2012 in the average number of territorial males recorded per route (Figure 4). The inflection point occurred in the early 1990s, coincident with a period of rapid expansion of pine silviculture in eastern Texas where plantations increased from 0.47 million ha in 1985 (USDA Forest Service 1988), to 0.74 million ha in 1992 (Rosson Reference Rosson2000), and 1.09 million ha in 2011 (Cooper and Bentley Reference Cooper and Bentley2012).
$$\bar x$$
= 11.5 ± 4.5). BBS data show a marked non-linear increase from 1980 to 2012 in the average number of territorial males recorded per route (Figure 4). The inflection point occurred in the early 1990s, coincident with a period of rapid expansion of pine silviculture in eastern Texas where plantations increased from 0.47 million ha in 1985 (USDA Forest Service 1988), to 0.74 million ha in 1992 (Rosson Reference Rosson2000), and 1.09 million ha in 2011 (Cooper and Bentley Reference Cooper and Bentley2012).
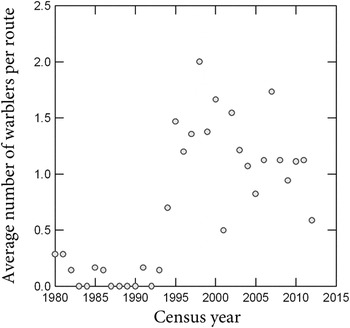
Figure 4. Average number of Swainson’s Warblers recorded per route on BBS census routes in eastern Texas from 1980 to 2012.
Reproduction in pine plantations
The only information on nesting productivity of Swainson’s Warbler in pine plantations originates from a limited area in south-eastern Louisiana where Henry (Reference Henry2004) showed that breeding density, clutch size, hatching rates, and reproductive success in pines were similar to those observed on adjacent plots in bottomland broadleaf forest of the Pearl River drainage. Moreover, reproductive success in Louisiana pine plantations (Henry Reference Henry2004) was roughly equivalent to estimates from broadleaf bottomland habitats in southern Missouri (Thomas et al. Reference Thomas, Wiggers and Clawson1996), eastern Arkansas (Benson et al. Reference Benson, Anich, Brown and Bednarz2010), and South Carolina (Bishop et al. Reference Bishop, Gerwin and Lancia2012). Although there are too little data to draw broad conclusions about nesting productivity in these fundamentally different habitats, there is no evidence thus far to suggest that nesting populations in pine plantations are any less productive than in broadleaf bottomland habitats or that pine plantations act as population sinks (Pulliam Reference Pulliam1988) or ecological traps (Battin Reference Battin2004).
Geographic extent of occupancy of pine plantations
The results of the present survey indicate that Swainson’s Warbler has not only extensively colonised pine plantations in eastern Texas (Carrie Reference Carrie1996) and south-eastern Louisiana (Henry Reference Henry2004, Bassett-Touchell and Stouffer Reference Bassett-Touchell and Stouffer2006, Legrand et al. Reference Legrand, Chamberlain and Moser2007), but has apparently expanded its breeding habitat niche to include pine plantations throughout its breeding range on the coastal plain from Texas to Virginia. Perhaps not coincidentally, the two peaks of current breeding abundance (2006–2011) according to BBS data occur in south-eastern Texas and central Alabama (http://www.mbr-pwrc.usgs.gov/bbs/ra2011/ra06380.htm) where the colonisation of pine plantations has been extensive (Figure 1). The presence of territories in pine plantations on the loess bluffs of Mississippi and in the piedmont areas of central Alabama suggest that the phenomenon may not be restricted to the coastal plain. However, the warbler has not been detected thus far in pine plantations in the Appalachian Mountains, on the Cumberland Plateau, or in the Ouachita or Ozark mountains.
Decadal trends in population distribution
Graves (Reference Graves2001) suggested that > 90% of breeding populations of Swainson’s Warbler occurred in broadleaf floodplain forest in the lower Mississippi Valley and on the coastal plain from eastern Texas to south-eastern Virginia. That calculation, however, was made more than a decade before the extent of the recent expansion of breeding populations into pine plantations was realised and before management changes that favoured maturation and restoration of broadleaf forests to steady-state conditions at the expense of early successional habitats were fully implemented on public lands in the south-eastern United States (LMVJV Forest Resource Conservation Group 2007, U.S. Fish and Wildlife Service 2010). Breeding populations of Swainson’s Warbler in the broadleaf bottomland forests on public lands in the lower Mississippi Valley and on the Gulf Coastal Plain have steadily decreased since the cessation of large-scale timber sales in the 1970s (author’s pers. obs.). As a prime example, several sites in White River drainage in eastern Arkansas that supported relatively dense breeding populations in the 1980s were completely devoid of breeding birds a decade later owing to the maturation of habitat and the consequent thinning of the understory.
Conclusions
Population limitation caused by the scarcity of early successional broadleaf habitat on the breeding grounds may be offset by the warbler’s recent and rapid colonisation of pine plantations. Behavioural plasticity in habitat selection may explain why Swainson’s Warbler has survived two centuries of intense forest clearing and habitat alteration while Bachman’s Warbler Vermivora bachmanii, which bred in similar microhabitats in the south-eastern United States (Wayne Reference Wayne1907, Hamel Reference Hamel1986), has become extinct. Southern pine silviculture has expanded into regional patchworks of even-aged plantations that now cover more than 15.8 million ha on the coastal plain from east Texas to Virginia. Given the 25–35 year rotation cycles commonly prescribed for private and commercial plantations, and a ∼7–8 year window of habitat suitability for Swainson’s Warbler in a typical stand, roughly a quarter of pine plantations will be in the appropriate seral stage at any given moment. Of broader significance, southern pine plantations are projected to increase to 27.1 million ha by 2060 (Wear and Greis Reference Wear and Greis2012). If current distributional trends continue, forestry lands managed for short-rotation pine plantations will soon support a majority of the global breeding population of Swainson’s Warbler.
Acknowledgements
I thank T. J. Benson, John Gerwin, and two anonymous reviewers for critiquing the manuscript and providing many helpful suggestions, the U.S. Fish and Wildlife Service, U.S. Department of Agriculture Forest Service, and the Anderson-Tully Corporation for access to lands. Brian Schmidt produced the base maps. Funding (1988–2014) was provided by the Research Opportunities Fund and the Alexander Wetmore Fund of the Smithsonian Institution, the U.S. Fish and Wildlife Service (14-48-0005-92-9013 and 14-48-0009-946) for surveys in the Great Dismal Swamp, and the Smoketree Trust. Funding sources had no influence on study design, analysis or interpretation of data, or article preparation.










