Breast cancer is the most prevalent cancer and the most common cause of death from cancer in women in the USA( Reference Siegel, Miller and Jemal 1 ). In 2013, approximately 21 % of newly diagnosed breast cancer cases were in situ breast cancer with 85 % of them as ductal carcinoma in situ (DCIS)( 2 ). DCIS starts with abnormal breast changes in the ductal cells and more than one-third of cases will develop into invasive cancer( 2 ). The progression from normal tissue to DCIS and to invasive cancer depends on many risk factors. Beyond conventional surgical treatment and neoadjuvant therapy, women with breast cancer often attempt to seek alternative treatment to improve outcomes. A report in 2002 showed that 63–83 % of breast cancer patients used at least one type of complementary and alternative medicine to prevent the development or progression of breast cancer( Reference DiGianni, Garber and Winer 3 ). Nutritional and dietary intake modifications were among the most commonly used complementary and alternative medicines( Reference DiGianni, Garber and Winer 3 ). Dietary factors, especially higher vegetable intake, appear to be associated with breast cancer prevention( Reference Rossi, Pericleous and Mandair 4 , Reference Liu, Huang and Gao 5 ), although the role of vegetable consumption in breast cancer risk remains controversial( Reference Smith-Warner, Spiegelman and Yaun 6 ). Cruciferous vegetables are a subgroup of vegetables of interest to researchers due to their potential cancer-preventive effects( Reference Abdull Razis and Noor 7 ). A recent meta-analysis including thirteen epidemiological studies showed that cruciferous vegetable intake is inversely associated with breast cancer( Reference Liu and Lv 8 ).
Cruciferous vegetables are named for their cross-shaped flower petals, and include bok choi, broccoli, Brussels sprouts, cabbage, cauliflower, collard greens, radishes, kale, mustard greens, sauerkraut, turnips and many others. The consumption of cruciferous vegetables is very different across cultures and countries. The average dark green and cruciferous vegetables consumption among US adults aged 25–75 years was only 0·2 serving daily, according to results from the 1994–1996 Continuing Survey of Food Intakes by Individuals conducted by the US Department of Agriculture( Reference Johnston, Taylor and Hampl 9 ). This average intake was far less than the intake of cruciferous vegetables of women in Asian countries, who had lower breast cancer incidence (e.g. the estimated median intake was 83 g/d among women participating in the Shanghai women’s study; n 73 360)( Reference Zhang, Shu and Xiang 10 ).
The major phytochemicals in cruciferous vegetables are glucosinolates, the precursors of bioactive isothiocyanates (ITC), which appear to modulate breast cancer risk at multiple stages of carcinogenesis through a variety of biological mechanisms. Sulforaphane (SFN) is an ITC derived from cruciferous vegetables and is especially high in broccoli and broccoli sprouts( Reference Fahey, Zhang and Talalay 11 , Reference Zhang, Talalay and Cho 12 ). A high intake of SFN may reduce breast cancer risk through inducing phase II detoxifying enzymes such as glutathione S-transferases, inducing apoptosis and decreasing cell proliferation( Reference Fahey, Zhang and Talalay 11 , Reference Gingras, Gendron and Boivin 13 ). It may also target epigenetic alterations and is an inhibitor of histone deacetylases (HDAC), leading to cell-cycle arrest and apoptosis( Reference Pledgie-Tracy, Sobolewski and Davidson 14 ). However, how cruciferous vegetable intake impacts these processes and tumour biomarkers in human breast tissue is unclear. No study has examined cruciferous vegetable and breast tissue biomarkers in vivo.
In the present study, we used the baseline data from a clinical trial of SFN supplementation. The trial was an eight-week, double-blinded, randomized, placebo-controlled study designed to examine effectiveness of supplemental SFN intake in improving biomarkers for cancer prognosis and in altering HDAC activity among women scheduled for breast biopsy. The purpose of the present analysis was to comprehensively examine the relationship between regular cruciferous vegetable intake and selected cancer prognosis-related biomarkers in women’s plasma and breast tissue samples before initiation of the SFN supplement intervention.
Methods
Participants
Participants were fifty-four women enrolled in a randomized controlled trial conducted at Oregon Health & Science University’s Center for Women’s Health Breast Center in Portland, OR, USA from August 2009 to December 2013 (Fig. 1). In brief, women were identified at the time they had suspicious mammographic findings that required breast biopsy at Oregon Health & Science University, Kaiser Permanente Northwest and Epic Imaging Clinics, or women from the public sector who found the study listed on clinicaltrials.gov.
English-speaking women were eligible to participate in the study if they were ≥21 years old and had a diagnostic mammogram with results that required biopsy. Exclusion criteria included: invasive breast cancer without DCIS or atypical ductal hyperplasia (ADH), pregnancy, breast-feeding, significant active medical illness, history of or active liver disease or baseline total bilirubin greater than the upper limit of the normal range, allergy to cruciferous vegetables, use of oral antibiotics (except for doxycycline) within three months prior to randomization, oral steroid therapy at enrolment, current therapy with valproate acid or suberoyl + anilide + hydroxamic acid (SAHA), current and planned continuous use of SFN-containing supplements, herbal remedies or pharmaceutical HDAC inhibitors, additional surgeries scheduled within 30 d of the study start date, neoadjuvant radiation or chemotherapy for currently diagnosed disease prior to or during study supplementation, or any condition possibly exacerbated by participation.
Eligible women met with study coordinators at the Oregon Health & Science University Clinical and Translational Research Center to review the study’s purpose and exclusion criteria. All participants provided informed consent. For the analysis reported in the present paper, only baseline data when participants were first enrolled in the clinical trial were used.
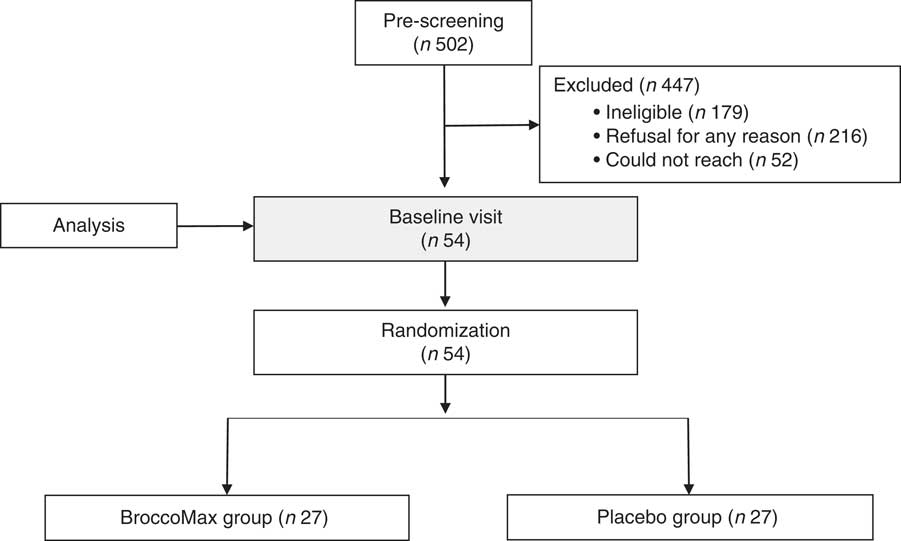
Fig. 1 Sample size flowchart
Data collection
Sociodemographic and lifestyle data were collected through a risk factor questionnaire administered by experienced study coordinators. A spot urine specimen was collected from each participant to rule out pregnancy prior to enrolment and was used to assess concentrations of SFN ITC. Each urine sample was aliquoted into seven small (1·0 ml) vials and labelled. A 30 ml non-fasting whole blood specimen was collected at baseline and processed for analysis of plasma SFN ITC concentrations, HDAC activity and acetylated histones. Paraffin-embedded breast biopsy tissue samples, which were regularly collected and stored as part of the surgical procedure, were requested for our analyses from participants’ respective institutional pathology laboratories. All breast biopsy tissues were clinically evaluated for the presence of DCIS and/or ADH or invasive cancer by a board certified pathologist. All biological samples were stored chronologically in a freezer at −80°C.
Diet and cruciferous vegetable intake assessment
Dietary intake was assessed using an interviewer-administered National Cancer Institute (NCI) Diet History Questionnaire (DHQ) and Arizona Cruciferous Vegetable Food Frequency Questionnaire (CVFFQ). The NCI DHQ asked questions regarding the participant’s usual dietary intake and the Arizona CVFFQ is a validated assessment tool used to specifically quantify cruciferous vegetable consumption( Reference Thomson, Newton and Graver 15 ). This questionnaire lists twenty cruciferous vegetables food lines (each food line asks respondents one specific kind of cruciferous vegetable consumption), six mixed-vegetable dishes and six condiments. It can be used to evaluate cruciferous vegetable intake more accurately than standard FFQ( Reference Thomson, Newton and Graver 15 ). All questionnaire data were hand-entered into a Clinical and Translational Research Center Bioinformatics-supported REDCap™ database. Data from the DHQ were analysed using Diet*Calc software. All the items listed on the CVFFQ were linked to the US Department of Agriculture’s National Nutrient Database for Standard Reference Release 27 as well as the Food and Nutrient Database for Dietary Studies (FNDDS) version 1·0; each item was given a weighted average based on national consumption data from the National Health and Nutrition Examination Survey. Weight in grams for individual ingredients of mixed dishes and condiments were derived from recipes in FNDDS( 16 ), as was the mean intake in grams of each cruciferous vegetable item listed in the questionnaire.
Measurement of isothiocyanate levels in urine and plasma
After centrifugation, plasma and urine supernatants were filtered twice through centrifuge tube filters. Plasma filtrates were stored at −80°C until further analysis. Urine filtrates were diluted 1:2 with 0·1 % (v/v) formic acid in H2O prior to storage. Plasma and urine SFN ITC levels were measured by HPLC–MS/MS at Oregon State University’s Mass Spectrometry Core Laboratories using the same materials, methods and instrumentation as previously described( Reference Clarke, Hsu and Riedl 17 ). Levels were analysed for SFN, SFN-cysteine (SFN-Cys), SFN-glutathione (SFN-GSH), SFN-cysteinylglycine (SFN-CG) and SFN-N-acetylcysteine (SFN-NAC) in duplicate following a 10 μl injection. Metabolite levels were quantified based on standard curves spanning metabolite concentrations. All urinary SFN ITC levels were adjusted for urine creatinine level.
Measurement of histone deacetylase activity in peripheral blood mononuclear cells
Analyses were performed by the Cancer Chemoprevention Program’s Core Laboratory at the Linus Pauling Institute. Peripheral blood mononuclear cells (PBMC) were isolated using Histopaque (Sigma, St. Louis, MO, USA) separation, suspended in dimethyl sulfoxide and stored in liquid nitrogen until further analysis. PBMC HDAC activity was evaluated using an in-house HDAC substrate Ac-K-Y-K(ac)-AMC. The deacetylated standard was custom synthesized by AAPPTec, LLC (Louisville, KY, USA). The intra-assay CV was 8·2 %. HDAC activity is expressed relative to protein content in lysate.
Measurement of biomarkers from breast tissues through immunohistochemistry
Immunohistochemistry was performed on paraffin-embedded breast biopsy specimens using methods described by Elsheikh et al.( Reference Elsheikh, Green and Rakha 18 ). Slides were prepared from each biopsy core using Fisher Plus slides. These slides were air-dried at 25°C then were further dried at 60°C in preparation for staining procedures. In order to be appropriately stained with primary antibodies for acetylated histone H3 at lysine 9 (H3K9) and 18 (H3K18), p21, Ki-67, HDAC6 and HDAC3, slides were deparaffinized in xylenes, rehydrated with graded alcohols, washed in Tris-buffered saline (pH 7·2–7·6), heated in a Russell-Hobbs programmable pressure cooker in 0·01-m citrate buffer (pH 6·0), treated with 3 % (w/v) aqueous H2O2 solution, blocked at 25°C in 3 % (w/v) goat serum and then incubated for 1 h at 25°C. After incubation with antibodies, the following procedures were performed: mouse EnVision, counterstaining with Gill’s hematoxylin, rinsing, treating with Tris-buffered saline, dehydration with graded alcohols and xylene, and cover slipping using Permount. Positive and negative control slides were used for technical adequacy of staining. Our collaborating pathologist read the slides and scored both staining intensity (graded as 1–3) and percentage of positive cells. A modified Histo-score (H-score) ranging from 0 to 300 was recorded( Reference McCarty, Miller and Cox 19 ).
Statistical analyses
Descriptive data on baseline characteristics of all the participants were expressed as means and standard deviations for continuous variables and as counts and percentages for categorical variables. The primary outcomes examined include urine and plasma SFN ITC levels, levels of acetylation at H3K9 and H3K18, HDAC activity, Ki-67 and p21. Kolmogorov–Smirnov normality tests were conducted for all continuous variables. HDAC activity in PBMC followed a normal distribution. Tissue biomarkers were not normally distributed and therefore were log2-transformed to obtain approximate normality. Total cruciferous vegetable intake, plasma and urine biomarkers were analysed untransformed.
Our primary interest was to determine whether total cruciferous vegetable intake, combining sources from food, mixed dishes and condiments, is significantly associated with the biomarkers of our primary interest, histone acetylation and cell proliferation. The analysis was conducted separately for each primary end point as well as for each specimen type (e.g. blood, urine, normal tissue, DCIS tissue and invasive ductal carcinoma (IDC) tissue). In order to examine the association between total cruciferous vegetable intake and each biomarker, we first conducted crude and partial Spearman correlation tests, followed by multivariable generalized linear regression analyses adjusting for potential confounders. To account for multiple comparisons, we conducted false discovery rate (FDR) calculation using the Benjamini and Hochberg method( Reference Benjamini and Hochberg 20 ). All statistical analyses were performed using the statistical software package SAS version 9·4. A FDR q value <0·05 was considered statistically significant.
Results
The baseline characteristics of all participants showed 74 % were white, 37 % had a family history of breast cancer, 26 % were either current or former smokers, 50 % drank alcohol, 22 % had income ≤$US 25 000, 11 % had ≤12 years of education, 50 % were married, 52 % were users of non-steroidal anti-inflammatory drugs, 43 % were postmenopausal women and 48 % were diagnosed with cancer (including DCIS or invasive disease). The mean age was 54·4 (sd 12·1) years among the fifty-four participants (Table 1).
Table 1 Sociodemographic characteristics of women participating in a randomized clinical trial (n 54)
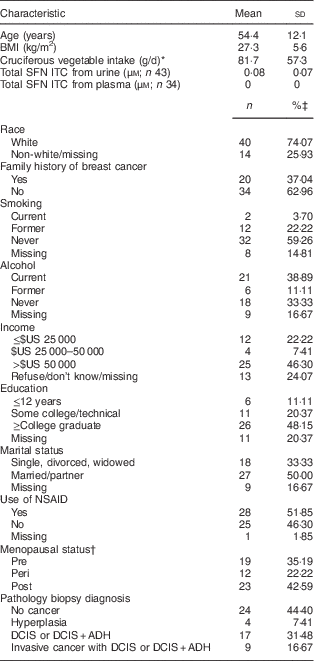
SFN ITC, sulforaphane isothiocyanates; NSAID, non-steroidal anti-inflammatory drugs; DCIS, ductal carcinoma in situ; ADH, atypical ductal hyperplasia.
* Cruciferous vegetable intake includes sources from food lines, mixed dishes and condiments.
† For the thirteen women who did not report their menopausal status, those aged <45 years were grouped into premenopausal status, those aged 45–55 years were grouped into perimenopausal status and those aged >55 years were grouped into postmenopausal status.
‡ Percentages may not add up to 100 due to rounding.
The mean baseline total cruciferous vegetable intake was 81·7 (sd 57·3) g/d among the total of fifty-four participants. Forty-three of them had data of urinary SFN ITC values and the average total SFN ITC value was 0·08 (se 0·01) µm/mm creatinine (Table 2). Crude (unadjusted) and partial (adjusted) Spearman correlation between total cruciferous vegetable intake and biomarkers are shown in Table 3. Partial Spearman correlation showed total cruciferous vegetable intake was significantly correlated with Ki-67 in DCIS (P=0·009) and IDC tissue (P=0·03), and was possibly correlated with p21 in DCIS tissue (P=0·06) after adjusting for age and use of non-steroidal anti-inflammatory drugs. In the multivariable regression analysis adjusting for age and use of non-steroidal anti-inflammatory drugs, total cruciferous vegetable intake was significantly inversely associated with Ki-67 in DCIS tissue (β=−0·004, se=0·001, FDR q value=0·03; Table 4). Additional adjustment for smoking or alcohol intake did not change the significant association. No association was found for other biomarkers including p21. Because the tissue-derived biomarkers were log2-transformed, β coefficients were interpreted as percentages. For Ki-67, a 1 g/d increase in cruciferous variable intake was associated with a 0·3 % decrease in Ki-67 level in DCIS tissue. As shown in Fig. 2, the levels of predicted Ki-67 H-score decreased dramatically with increasing total cruciferous vegetable intake, especially after intake of 60 g/d. In exploratory analyses, the relationship between cruciferous vegetable intake and Ki-67 level appeared to be non-linear with a threshold at an intake of about 60 g/d. The greatest evidence for an association between cruciferous vegetable intake and Ki-67 level was noted above this threshold. For this reason, we categorized the participants into two groups based on their total cruciferous vegetable intake: (i) ≥60 g/d (higher cruciferous vegetable intake, n 33); and (ii) <60 g/d (lower cruciferous vegetable intake, n 21). The association between cruciferous vegetable intake and Ki-67 level was significant among individuals in the higher cruciferous vegetable intake category (β=−0·04, se=0·0, P=0·04). For those in the lower cruciferous vegetable intake, we did not find a significant association between cruciferous vegetable intake and Ki-67 level (β=−0·04, se=0·09, P=0·70). However, the magnitude of the association was the same in both groups (β=−0·04).
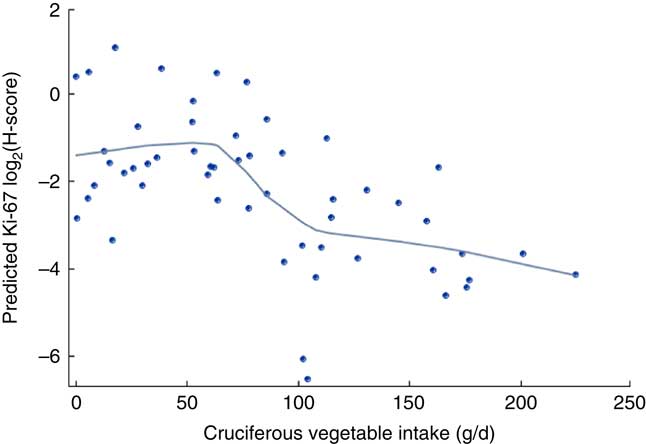
Fig. 2 Ki-67 modified Histo-score (H-score) levels in breast ductal carcinoma in situ tissue and cruciferous vegetable intake among women (n 54) at high risk for breast cancer participating in a randomized clinical trial. Predicted Ki-67 log2(H-score) levels (![]() ;
; ![]() , loess smooth=0·5) are adjusted for age and use of non-steroidal anti-inflammatory drugs. Figure was obtained through SGPLOT procedure in SAS version 9·4
, loess smooth=0·5) are adjusted for age and use of non-steroidal anti-inflammatory drugs. Figure was obtained through SGPLOT procedure in SAS version 9·4
Table 2 Baseline daily intake of selected cruciferous vegetables and urinary sulforaphane metabolites among women participating in a randomized clinical trial
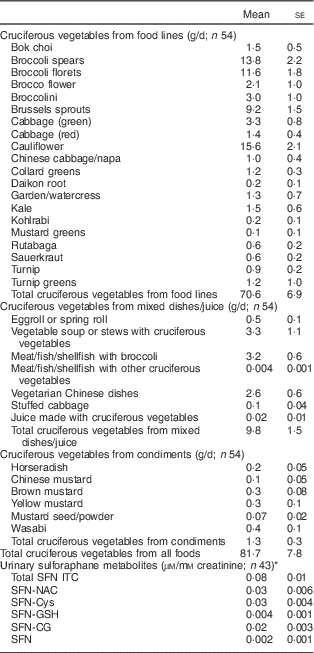
SFN ITC, sulforaphane isothiocyanates; SFN-NAC, SFN-N-acetylcysteine; SFN-Cys, SFN-cysteine; SFN-GSH, SFN-glutathione; SFN-CG, SFN-cysteinylglycine; SFN, sulforaphane.
* Urinary metabolite concentrations normalized to creatinine in millimolar concentration.
Table 3 Spearman correlations between total cruciferous vegetable intake and blood as well as tissue biomarkers among women at high risk for breast cancer participating in a randomized clinical trial

PBMC, peripheral blood mononuclear cells; HDAC, histone deacetylase; ac, acetylation; DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; na, not available.
Significant results are presented in bold font.
* Partial correlation P value is adjusted for age and use of non-steroidal anti-inflammatory drugs.
Table 4 Multivariable regression analysis between total cruciferous vegetable intake and blood as well as tissue biomarkers among women at high risk for breast cancer participating in a randomized clinical trial
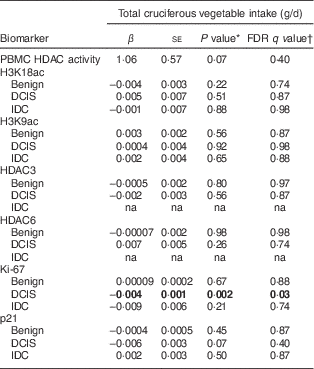
FDR, false discovery rate; PBMC, peripheral blood mononuclear cells; HDAC, histone deacetylase; ac, acetylation; DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; na, not available; H-score, modified Histo-score.
Significant results are presented in bold font.
* General linear regression models were used, adjusting for age and use of non-steroidal anti-inflammatory drugs. The units of H3K18ac, H3K9ac, HDAC3, HDAC6, Ki-67 and p21 used in the model were log2(H-score).
† Multiple comparison-adjusted FDR q values for all the breast-tissue immunohistochemistry biomarkers using the Benjamini–Hochberg False Discovery Rate.
Discussion
In the current analysis of our fifty-four female participants, we found that total cruciferous vegetable intake was associated with a lower level of Ki-67 in DCIS tissues. We did not observe a statistically significant association between total cruciferous vegetable intake and H3K18ac, H3K9ac, HDAC3, HDAC6 or p21 from the limited sample size.
Although numerous studies have assessed the effects of cruciferous vegetable intake on breast cancer, the relationship between cruciferous vegetable intake and blood or breast tissue biomarkers has received less attention. In a cross-sectional analysis among 1005 middle-aged Chinese women from the Shanghai Women’s Health Study, Jiang et al. found that cruciferous vegetable intake was inversely associated with circulating levels of pro-inflammatory markers such as TNF-α, IL-1β and IL-6( Reference Jiang, Wu and Shu 21 ). The median intake of cruciferous vegetables was 82·9 g/d with an interquartile range of 48·9–138·1 g/d( Reference Jiang, Wu and Shu 21 ), which was a little higher than the median intake of cruciferous vegetables in our study population (median=74·5 g/d, interquartile range=32·2–114·6 g/d). The most commonly consumed cruciferous vegetables in China included Chinese greens (bok choi), green cabbage, Chinese cabbage (napa), cauliflower and white turnip/radish( Reference Jiang, Wu and Shu 21 ), while the five most commonly consumed cruciferous vegetables in our study population were cauliflower, broccoli spears, broccoli florets, Brussels sprouts and broccolini. Therefore, there was a difference in the food patterns of cruciferous vegetable consumption between these populations. However, no study has examined cruciferous vegetable intake and breast tissue biomarkers in vivo. Our finding of the statistically significant inverse association between total cruciferous vegetable intake and Ki-67 in DCIS tissue is biologically plausible. Ki-67 is a biomarker for cell proliferation. Cell proliferation is a hallmark of cancer, although the level of proliferation differs along cancer disease stages. How breast tissue cells respond to cruciferous vegetable intake and their molecular derivatives at different stages is not well studied. Our report is the first study showing the differential effects of cruciferous vegetable intake, the main source of SFN, on Ki-67 in IDC, DCIS and benign breast tissue. Our observations support the notion that cancer disease stage, including both breast cancer and other types of cancer stage, may influence a cell’s response to cruciferous vegetable intake. An in vitro study on prostate cells also showed SFN had different effects on cell-cycle arrest and apoptosis in different stages of cells when comparing normal v. hyperplastic and cancerous prostate cells( Reference Clarke, Hsu and Yu 22 ).
Based on our study’s exclusion criteria, anyone who took SFN supplements before enrolment was not eligible. Therefore, our analyses of all the baseline (before intervention) plasma SFN ITC levels including total SFN ITC, SFN-NAC, SFN-Cys SFN-GSH and SFN-CG were 0, indicating people were not taking SFN supplements and all the participants enrolled met the non-SFN supplement criterion. Our study did not find a statistically significant correlation between usual cruciferous vegetable intake and urinary SFN ITC as we expected. This finding is understandable given that urinary SFN ITC levels reflect recent intake of SFN, not usual cruciferous vegetable intake, as SFN ITC are eliminated from urine within 48 h after cruciferous vegetable intake( Reference Kristensen, Krogholm and Frederiksen 23 ). One large study of two cohorts in China, including 3589 women and 1015 men, showed self-reported usual dietary cruciferous vegetable intake was weakly correlated with urinary ITC level (r=0·1149, P<0·0001), while recent dietary cruciferous vegetable intake was more strongly correlated with urinary ITC level (r=0·2591, P<0·0001)( Reference Vogtmann, Yang and Li 24 ).
In our analysis of the relationship between total cruciferous vegetable intake and PBMC HDAC activity, we did not observe a significant correlation. This result is outside our expectations, as we hypothesized the cruciferous vegetable intake could decrease HDAC activity. We conducted a sensitivity analysis investigating the relationship between PBMC HDAC and urinary total SFN ITC, and observed a non-significant negative relationship (β=−505·7, se=493·6, P=0·31). The non-significant finding between total cruciferous vegetable intake and PBMC HDAC activity could be due to chance and small sample size.
Genetic factors should be taken into consideration when studying the association of cruciferous vegetable intake and breast cancer risk. Only a few studies have investigated the role of genes in this association. One study using data from the Long Island Breast Cancer Study, a population-based case–control study, reported no interaction between genetic polymorphisms of glutathione S-transferases, which are phase II enzymes that metabolize various bioactive compounds, and cruciferous vegetable intake in regard to cancer risk( Reference Steck, Gaudet and Britton 25 ). To date, we have not conducted genetic testing on collected blood; therefore, we have not assessed the effects of genetic polymorphisms in our study.
Human gut microbiota is another factor that should be considered when directly evaluating the effect of cruciferous vegetable intake on breast cancer or its related biomarkers. Glucosinolates in cruciferous vegetables can be metabolized to ITC by plant myrosinase or by certain bacteria in the human gut. Previous studies have observed individual differences in glucosinolate metabolism( Reference Rouzaud, Young and Duncan 26 – Reference Shapiro, Fahey and Wade 28 ) and the human gut microbial community could contribute to the individual variation( Reference Rouzaud, Young and Duncan 26 , Reference Li, Hullar and Beresford 27 ). To date, only one human study has examined the relationship between cruciferous vegetable intake, ITC production and gut microbiota composition among healthy adults( Reference Li, Hullar and Beresford 27 ). Among these participants who consumed the same amount of cooked broccoli from a standardized meal, the authors found a significant difference in glucosinolate degradation by fecal bacteria between participants with high and low urinary ITC excretion( Reference Li, Hullar and Beresford 27 ). No study has examined the impact of gut microbiota on the effect of cruciferous vegetable intake on cancer biomarkers.
Our study has several strengths. First, we have comprehensive data on biomarkers from urine and blood, and we collected breast biopsy tissue of each participant. We retain the capacity to examine the associations between total cruciferous vegetable intake and biomarkers in the remaining tissue. To our knowledge, our study is the first one examining associations between cruciferous vegetable intake and breast tissue biomarkers in human subjects. Second, cruciferous vegetable intake was assessed specifically by a validated cruciferous vegetable intake questionnaire that has better accuracy in calculating total cruciferous vegetable intake than conventional FFQ. Study limitations include the small sample size of participants and the smaller sample size of available breast tissue specimens. Other limitations include the hospital-based study design, which may limit the generalizability of our results. In addition, the median intake of cruciferous vegetables among participants in our study was higher compared with the general US population. This is within our expectation since our study participants came from a dietary supplement intervention trial, who were more motivated to eat cruciferous vegetables. Therefore, our results may not be generalized to the entire US population.
Conclusion
Our data suggest that cruciferous vegetable intake is associated with decreased cell proliferation rate indicated by Ki-67 level. From a nutrition perspective, these data reinforce the recommendation of cruciferous vegetable intake in breast cancer prevention. Our results also provide further guidance for individuals to increase their cruciferous vegetable intake, especially among women diagnosed with DCIS, in order to prevent breast cancer cell proliferation. Future population-based studies with larger numbers of participants are needed to increase the generalizability of the study results.
Acknowledgements
Acknowledgements: The authors are grateful to all the women who took the time to participate in the study (Sulforaphane: A Dietary HDAC Inhibitor and Prevention of DCIS Progression). Sincere appreciation also goes to Dr Megan Troxell for conducting immunohistochemical analyses for this study, Dr David Yu for performing HDAC inhibition assays and Mr Vernon L. Hartz for providing advice on cruciferous vegetable analysis. Financial support: Research reported in this publication was supported by the National Cancer Institute (grant numbers R21 CA132236-01A2 and P01 CA090890); the National Center for Advancing Translational Sciences of the National Institutes of Health (grant number UL1TR000128); and the National Institute of Environmental Health Sciences (grant number P30 ES000210). Clinical Trial Registration: clinicaltrials.gov identifier is NCT00843167. The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: Z.Z. conducted statistical analyses, interpreted the data and wrote the first draft of the manuscript; L.L.A. assessed all the biomarkers from urine and blood, and revised the manuscript; J.S., E.H. and P.E.F. conceptualized, designed the study and collected the data; all authors read and revised the draft, and approved the final manuscript. Ethics of human subject participation: This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving participants were approved by the Oregon Health & Science University and Kaiser Permanente Northwest Institutional Review Boards for the protection of human subjects. Written informed consent was obtained from all participants.











