Liver, the largest of solid organs in the body, is involved in the crucial detoxification process( Reference Gao, Jeong and Tian 1 ), besides other important functions. In addition, the liver is also an important part of the body's immune response, and is therefore considered an immunological organ( Reference Racanelli and Rehermann 2 ), which is selectively enriched in macrophages (Kupffer cells). However, multiple factors such as inflammatory stimuli and microbial products, induce Kupffer cells to produce various pro-inflammatory cytokines, such as IL-1β, IL-6 and TNF-α. Consequently, over-production of these pro-inflammatory cytokines leads to liver injury and liver dysfunction( Reference Chen, Liu and Zhu 3 , Reference Schmöcker, Weylandt and Kahlke 4 ). Among these cytokines, TNF-α is the central mediator of hepatotoxicity in many models of hepatic damage, especially those involving the lipopolysaccharide (LPS)( Reference Bradham, Plümpe and Manns 5 ).
Much evidence suggests that innate immunity can specifically detect infection through pattern-recognition receptors that recognise pathogen-associated molecular patterns, which are expressed by invading pathogens. Transmembrane Toll-like receptors (TLR) and cytoplasmic nucleotide-binding oligomerisation domain proteins (NOD) are two major forms of innate pattern-recognition receptors( Reference Fukata, Vamadevan and Abreu 6 , Reference Takeuchi and Akira 7 ). Among the TLR family, TLR4 has been detected on all types of liver cells including Kupffer cells, and is activated by a low dose of LPS( Reference Szabo, Dolganiuc and Mandrekar 8 , Reference Schwabe, Seki and Brenner 9 ). NOD1 and NOD2 were specialised NOD reported to have a direct function as pattern-recognition receptors, in the recognition of peptidoglycan and LPS( Reference Moreira and Zamboni 10 ). NOD1 and NOD2 are also highly expressed in liver cells( Reference Scott, Chen and Sun 11 , Reference Swain, Basu and Samanta 12 ). Interaction of pathogen-associated molecular patterns with TLR or NOD triggers downstream signalling events that lead to the activation of NF-κB, which then stimulates the expression of inflammatory genes, including TNF-α, IL-1β and IL-6( Reference Fukata, Vamadevan and Abreu 6 , Reference Takeuchi and Akira 7 ). Consequently, the over-expression of pro-inflammatory cytokines elicits collateral host-tissue injury, especially liver injury.
Asparagine (Asn) is a neutral amino acid. Traditionally, Asn is thought as a nutritionally non-essential amino acid in mammals( Reference Wu 13 , Reference Wu, Bazer and Davis 14 ). However, increasing evidence has shown that Asn plays a significant role in immune function( Reference Li, Yin and Li 15 ). Suzuki et al. ( Reference Suzuki, Okayasu and Tashiro 16 , Reference Suzuki, Tashiro and Hashimoto 17 ) reported that LPS challenge led to macrophages activation, and it stimulated the production of Asn, NO, citrulline and the consumption of arginine by macrophages. These data suggest the possible link between the activation of macrophages and Asn production, and also suggest a positive relationship between the extent of Asn production and that of NO or citrulline. In the liver, constitutively generated NO maintains the hepatic microcirculation and endothelial integrity, and thereby minimises liver injury( Reference Chen, Zamora and Zuckerbraun 18 , Reference Ito, Abril and Bethea 19 ). However, to our knowledge, little research has been conducted to investigate the effect of Asn on liver.
Based on the findings of the studies cited abve, LPS-induced liver injury is characterised by local and systemic inflammation, which is related to Kupffer cell activation via activating TLR4 and NOD signalling pathways( Reference Chen, Liu and Zhu 3 ). So, we hypothesised that Asn might affect TLR4 and NOD signalling pathways, and exert a beneficial effect on liver integrity. In the present study, we employed a piglet model, an excellent animal model for studying human nutrition and physiology( Reference Puiman and Stoll 20 , Reference Merrifield, Lewis and Claus 21 ). The purpose of the present study was to evaluate the effect of dietary Asn supplementation on LPS-induced liver injury, and to elucidate its molecular mechanism(s). Our findings, we hope, will not only help to elucidate the role of Asn in the liver injury of piglets, but also provide new perspectives on developing new nutritional interventions to mitigate human liver damage in inflammatory condition.
Materials and methods
Pig care and experimental design
The present study was approved by the Animal Care and Use Committee of Hubei Province, China. A total of forty-eight castrated barrows (Duroc × Large White × Landrace; 8·12 (se 0·56) kg initial body weight (BW)) were randomly allotted to four groups (n 12). Pigs were individually caged in 1·80 × 1·10 m pens of an environmentally controlled nursery barn. Piglets were allowed ad libitum access to feed and water. The basal diet (see online Supplementary Table S1) was formulated to meet or exceed National Research Council( 22 ) requirements for all nutrients. The amino acid concentration in feeds was analysed according to Wu et al. ( Reference Wu, Davis and Flynn 23 ). The room temperature was maintained at 25–27°C. Lighting was natural.
Treatments included: (1) non-challenged control (CONTR; pigs were fed a control diet and injected with 0·9 % sterile saline); (2) LPS-challenged control (LPSCC; pigs were fed with the same control diet and injected intraperitoneally with Escherichia coli LPS); (3) LPS+0·5 % Asn treatment (pigs were fed with a 0·5 % Asn diet and injected with LPS); and (4) LPS+1·0 % Asn treatment (pigs were fed with a 1·0 % Asn diet and injected with LPS). The doses of Asn (l-asparagine, purity >99 %; Amino Acid Bio-Chemical Company Limited) were used according to our previous study, which showed that dietary supplementation of 0·5 or 1·0 % Asn alleviated weight loss caused by LPS challenge in weanling pigs( Reference Li, Liu and Shi 24 ). To obtain isonitrogenous diets, 1·35, 0·68 and 0 % alanine (purity >99 %; Amino Acid Bio-Chemical Company Limited) were added to the control, 0·5 and 1·0 % Asn diets, respectively. After 19 d feeding with control, 0·5 and 1·0 % Asn diets, the challenged group was injected intraperitoneally with E. coli LPS (E. coli serotype 055:B5; Sigma Chemical, Inc.) at 100 μg/kg BW, and the unchallenged group received an equivalent amount of sterile saline. The dose of LPS (100 μg/kg BW) was chosen according to our previous studies( Reference Chen, Liu and Zhu 3 , Reference Wu, Davis and Flynn 23 , Reference Li, Liu and Che 25 ). After LPS or saline injection, all pigs were fed the same amount of feed/kg BW until slaughter in order to avoid the potential effects of LPS-induced feed intake reduction on blood and liver variables. The amount of feed/kg BW was determined according to the feed intake of LPSCC pigs during 24 h LPS challenge. The pigs were allowed ad libitum access to water.
Blood and liver sample collections
At 4 h after LPS or saline injection, six pigs (n 6) were selected randomly from each treatment group, humanely killed, and blood and liver samples were collected. At 24 h after LPS or saline injection, the remaining six pigs (n 6) were humanely killed to collect blood and liver samples. The procedures of blood and liver sample collections were according to our previous studies( Reference Chen, Liu and Zhu 3 ). Blood samples were centrifuged (3500 g for 10 min) to separate serum which was stored at − 80°C until analyses of serum biochemical parameters. One fragment of liver samples was fixed in fresh 4 % paraformaldehyde/PBS at least for 24 h, and embedded in paraffin. The remaining portions were rapidly frozen in liquid N2, and then stored at − 80°C for further analysis.
Liver morphology
Excised liver specimens (0·5 cm3) were fixed in 4 % paraformaldehyde/PBS for 24 h, dehydrated using a graded series of ethanol (70–100 %), cleared with xylene, and embedded in paraffin. Consecutive sections of 5 μm thickness were sliced and stained with haematoxylin and eosin for microscopic examination. Histological analysis was performed in a blinded manner using a light microscope with a computer-assisted morphometric system (BioScan Optimetric; BioScan) by an experienced pathologist.
Serum biochemical parameters
The activities of serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), glutamyl transpeptidase (GGT) and alkaline phosphatase (AKP) were determined according to Chen et al. ( Reference Chen, Liu and Zhu 3 ).
Liver TNF-α concentration and nitric oxide synthase activities
The TNF-α concentration in liver supernatant was determined using a commercially available porcine ELISA kit (REF: PTA00; R&D Systems). The minimum detectable dose of TNF-α was 3·7 pg/ml. The result of TNF-α was expressed as pg/mg protein. The total NO synthase (NOS) (tNOS) and inducible NOS (iNOS) activities were analysed using a commercially available assay kit (REF: A014-1; Nanjing Jiancheng Biological Product) in accordance with the instructions of the manufacturer. The results of tNOS and iNOS were calculated as U/mg protein. One enzyme activity unit (U) was defined as 1 nmol substrate generated per min under the assay conditions.
Western blotting measurement
The methods for protein immunoblot analysis were according to Chen et al. ( Reference Chen, Liu and Zhu 3 ). Briefly, hepatic samples (0·15–0·20 g) were homogenised in lysis buffer, and centrifuged to collect the supernatants for Western blot. An equal amount of hepatic proteins (65 μg) was loaded onto an SDS-PAGE, and then transferred to polyvinylidene difluoride membranes. The membranes were blocked for at least 60 min with 5 % non-fat milk in Tris-HCl-buffered saline (TBS)/Tween-20 buffer at room temperature, and then incubated overnight (12–16 h) at 4°C with primary antibodies. The membranes were incubated with the secondary antibody for 120 min at room temperature. Specific primary antibodies included rabbit anti-claudin-1 (REF: 519000, 1:1000; Invitrogen Technology, Inc.), mouse anti-HSP70 (REF: SPA-810, 1:1000; Stressgen) and mouse anti-β-actin (REF: A2228, 1:10 000; Sigma-Aldrich, Inc.). The secondary antibody included goat anti-rabbit IgG-HRP (REF: ANT020, 1:5000; Antgene Biotech) and goat anti-mouse IgG-HRP (REF: ANT019, 1:5000; Antgene Biotech). Blots were developed using an Enhanced Chemiluminescence Western blotting kit (Amersham Biosciences), and visualised using a Gene Genome bioimaging system. Bands were analysed by densitometry using GeneTools software (Syngene). The relative abundances of claudin-1 and heat shock protein 70 (HSP70) protein were expressed as claudin-1 protein:β-actin protein ratio and HSP70 protein:β-actin protein ratio, respectively.
mRNA expression analysis by real-time PCR
Total RNA isolation, quantification, complementary DNA synthesis and real-time PCR were carried out as previously described(
Reference Chen, Liu and Zhu
3
). The primer pairs used are shown in Table S2 (available online). The expression of the target genes relative to housekeeping gene (glyceraldehyde-3-phosphate dehydrogenase; GAPDH) was analysed by the
![]() $$2^{ - \Delta \Delta C _{T}} $$
method(
Reference Livak and Schmittgen
26
). GAPDH did not exhibit any difference among four treatments. Relative mRNA abundance of each target gene was normalised to the CONTR group.
$$2^{ - \Delta \Delta C _{T}} $$
method(
Reference Livak and Schmittgen
26
). GAPDH did not exhibit any difference among four treatments. Relative mRNA abundance of each target gene was normalised to the CONTR group.
Statistical analysis
All data were analysed using the general linear model procedure of statistical analysis system appropriate for a factorial arrangement of treatments in a randomised complete block design (SAS Institute Inc.). The statistical model included the effects of treatment (CONTR, LPSCC, LPS+0·5 % Asn and LPS+1·0 % Asn), time (4 or 24 h) and their interactions. Only when a significant treatment × time interaction occurred, comparisons among treatments in each sampling time (4 or 24 h) were performed. The following orthogonal contrasts were used to test treatment effects: (1) LPSCC v. CONTR was used to determine the response to LPS challenge; (2) linear and quadratic polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged piglets. Differences were considered as significant at P≤ 0·05, 0·05 < P≤ 0·10 were discussed as trends.
Results
Liver morphology
Regarding hepatic morphology, at 4 or 24 h post-injection, no obvious changes were observed in the livers of the CONTR pigs (Figs. 1(A) and 2(A)). However, morphologic changes associated with liver injury such as hepatocyte caryolysis, karyopycnosis, inflammatory cell infiltration, hepatocyte vacuolisation and hepatic cell cord arrangement in disorder were observed in LPSCC pigs (Figs. 1(B) and 2(B)). Compared with LPSCC pigs, liver injury was attenuated in the LPS+0·5 % Asn pigs (Figs. 1(C) and 2(C)) or LPS+1·0 % Asn pigs (Figs. 1(D) and 2(D)). Compared with lower concentration (0·5 %), higher concentration of Asn (1·0 %) had better protective effect on liver injury.
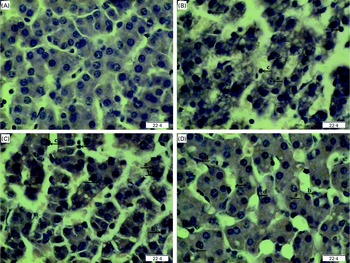
Fig. 1 Effects of asparagine (Asn) supplementation on liver morphology after 4 h lipopolysaccharide (LPS) challenge in weaned piglets. The representative photomicrographs of liver sections stained with haematoxylin and eosin are shown. (A) Non-challenged control (CONTR; pigs received a control diet and were injected with 0·9 % sterile saline). No obvious changes were found. (B) LPS-challenged control (LPSCC; pigs received the same control diet and were challenged with LPS). Morphologic changes associated with liver injury, such as (a) hepatocyte caryolysis, (b) karyopycnosis, (c) inflammatory cell infiltration, hepatocyte vacuolisation and hepatic cell cords arrangement in disorder, were observed. (C) LPS+0·5 % Asn treatment (pigs received a 0·5 % Asn diet and were challenged with LPS). (D) LPS+1·0 % Asn treatment (pigs received a 1·0 % Asn diet and were challenged with LPS), (d) fibroblast proliferation was observed. Liver injury was attenuated in LPS-challenged pigs fed 0·5 or 1·0 % Asn. Compared with 0·5 % Asn, 1·0 % Asn had better protective effect on liver injury. Original magnifications 400 × . Scale bars = 22·4 μm. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
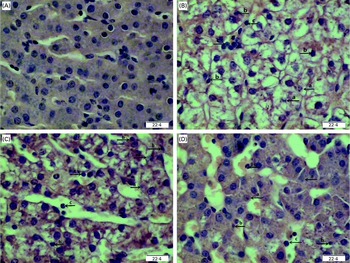
Fig. 2 Effects of asparagine (Asn) supplementation on liver morphology after 24 h lipopolysaccharide (LPS) challenge in weaned pigs. The representative photomicrographs of liver sections stained with haematoxylin and eosin are shown. (A) Non-challenged control (CONTR; pigs received a control diet and were injected with 0·9 % sterile saline). No obvious changes were found. (B) LPS-challenged control (LPSCC; pigs received the same control diet and were challenged with LPS). Morphologic changes associated with liver injury, such as (a) hepatocyte caryolysis, (b) karyopycnosis, (c) inflammatory cell infiltration, hepatocyte vacuolisation and hepatic cell cords arrangement in disorder, were observed. (C) LPS+0·5 % Asn treatment (pigs received a 0·5 % Asn diet and were challenged with LPS). (D) LPS+1·0 % Asn treatment (pigs received a 1·0 % Asn diet and were challenged with LPS). Liver injury was attenuated in LPS-challenged pigs fed 0·5 or 1·0 % Asn. Compared with 0·5 % Asn, 1·0 % Asn had better protective effect on liver injury. Original magnifications 400 × . Scale bars = 22·4 μm. A colour version of this figure can be found online at http://www.journals.cambridge.org/bjn
Serum biochemical parameters
Significant treatment × time interactions were observed for serum AST and AKP activities (P< 0·05; Table 1). At 4 h, compared to the CONTR piglets, LPS challenge increased serum AKP activity (P< 0·05).
Table 1 Effects of asparagine (Asn) supplementation on serum biochemical parameters after 4 or 24 h Escherichia coli lipopolysaccharide (LPS) challenge in weanling piglets (Mean values with their pooled standard errors, n 6)
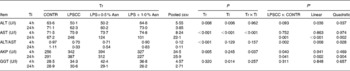
Tr, Treatment; Ti, Time; CONTR, non-challenged control (piglets received a control diet and were injected with 0·9 % sterile saline); LPSCC, LPS-challenged control (piglets received the same control diet and were injected with Escherichia coli LPS); LPS+0·5 % Asn, piglets received a 0·5 % Asn diet and were injected with LPS; LPS+1·0 % Asn, piglets received a 1·0 % Asn diet and were injected with LPS; ALT, alanine aminotransferase; AST, aspartate aminotransferase; AKP, alkaline phosphatase; GGT, glutamyl transpeptidase.
* LPSCC v. CONTR was used to determine the response to LPS challenge. Linear and quadratic polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged piglets.
At 24 h, relative to the CONTR piglets, LPS challenge increased serum AST and AKP activities (P< 0·05). With increasing Asn supplementation, serum AST and AKP activities were decreased (linear and quadratic, P< 0·01) in LPS-challenged pigs.
No significant treatment × time interaction was observed for serum ALT, ALT/AST and GGT. Overall, relative to the CONTR piglets, LPS challenge decreased serum ALT:AST ratio (P< 0·05), and tended to decrease serum ALT activity (P= 0·093). With increasing Asn supplementation, serum ALT activity and ALT:AST ratio were increased (linear and quadratic, P< 0·05) in LPS-challenged pigs. Neither LPS nor Asn supplementation had effect on serum GGT activity.
Liver claudin-1 protein expression
A trend for treatment × time interaction was observed for liver claudin-1 protein expression (P= 0·065; Fig. 3). At 4 h, relative to the CONTR piglets, LPS challenge decreased liver claudin-1 protein expression (P <0·05).
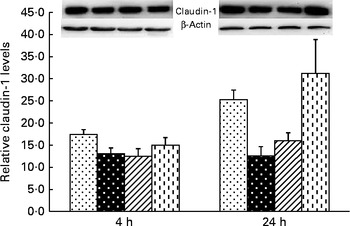
Fig. 3 Effects of asparagine (Asn) supplementation on protein expression of claudin-1 in liver after 4 or 24 h Escherichia coli lipopolysaccharide (LPS) challenge in weaned pigs. Relative level of claudin-1 was expressed as claudin-1 protein:β-actin protein ratio. Values are means (n 6), with their standard errors represented by vertical bars. Non-challenged control (CONTR (![]() ); pigs received a control diet and were injected with 0·9 % sterile saline; LPS-challenged control (LPSCC (
); pigs received a control diet and were injected with 0·9 % sterile saline; LPS-challenged control (LPSCC (![]() ); pigs received the same control diet and were challenged with LPS); LPS+0·5 % Asn (
); pigs received the same control diet and were challenged with LPS); LPS+0·5 % Asn (![]() , pigs received a 0·5 % Asn diet and were challenged with LPS); LPS+1·0 % Asn (
, pigs received a 0·5 % Asn diet and were challenged with LPS); LPS+1·0 % Asn (![]() , pigs received a 1·0 % Asn diet and were challenged with LPS). LPSCC v. CONTR was used to determine the response to LPS challenge. Linear (L) and quadratic (Q) polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged piglets. A trend for treatment × time interaction was observed for protein expression of liver claudin-1 (P= 0·065). At 4 h: LPSCC v. CONTR (P= 0·030), L (P= 0·390), Q (P= 0·518) and at 24 h: LPSCC v. CONTR (P= 0·002), L (P= 0·012), Q (P= 0·029).
, pigs received a 1·0 % Asn diet and were challenged with LPS). LPSCC v. CONTR was used to determine the response to LPS challenge. Linear (L) and quadratic (Q) polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged piglets. A trend for treatment × time interaction was observed for protein expression of liver claudin-1 (P= 0·065). At 4 h: LPSCC v. CONTR (P= 0·030), L (P= 0·390), Q (P= 0·518) and at 24 h: LPSCC v. CONTR (P= 0·002), L (P= 0·012), Q (P= 0·029).
At 24 h, relative to the CONTR piglets, LPS challenge decreased liver claudin-1 protein expression (P <0·01). With increasing Asn supplementation, protein expression of liver claudin-1 was increased (linear and quadratic, P< 0·05) in LPS-challenged pigs.
Liver mRNA and protein abundances of TNF-α and heat shock protein 70
Significant treatment × time interactions were observed for liver TNF-α and HSP70 mRNA abundance (P< 0·01; Table 2), and HSP70 protein abundance (P< 0·001; Fig. 4). At 4 h, compared to the CONTR piglets, LPS challenge increased liver TNF-α and HSP70 mRNA abundance, and TNF-α protein concentration (P< 0·05). With increasing Asn supplementation, liver TNF-α mRNA abundance (linear and quadratic, P< 0·05) was found decreased, and liver HSP70 mRNA abundance (linear, P= 0·067; quadratic, P= 0·087) and protein abundance (quadratic, P= 0·098) tended to be decreased in LPS-challenged pigs.
Table 2 Effects of asparagine (Asn) supplementation on liver mRNA or protein expression of TNF-α and heat shock protein 70 (HSP70) after 4 or 24 h Escherichia coli lipopolysaccharide (LPS) challenge in weanling piglets (Mean values with their pooled standard errors, n 6)
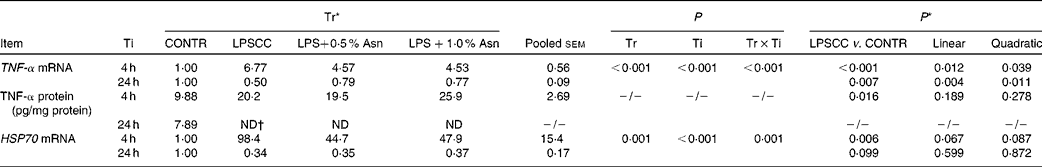
Tr, Treatment; Ti, Time; CONTR, non-challenged control (piglets received a control diet and were injected with 0·9 % sterile saline); LPSCC, LPS-challenged control (piglets received the same control diet and were injected with Escherichia coli LPS); LPS+0·5 % Asn, piglets received a 0·5 % Asn diet and were injected with LPS; LPS+1·0 % Asn, piglets received a 1·0 % Asn diet and were injected with LPS; ND, not detectable.
* LPSCC v. CONTR was used to determine the response to LPS challenge. Linear and quadratic polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged piglets.
† Lower than the minimum detectable dose of TNF-α.
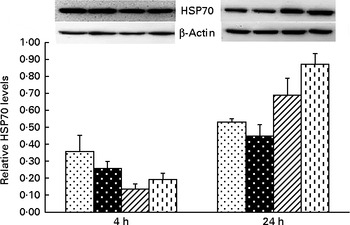
Fig. 4 Effects of asparagine (Asn) supplementation on protein expression of heat shock protein 70 (HSP70) in liver after 4 or 24 h Escherichia coli lipopolysaccharide (LPS) challenge in weaned pigs. Relative level of HSP70 was expressed as HSP70:β-actin protein ratio. Values are means (n 6), with their standard errors represented by vertical bars. Non-challenged control (CONTR (![]() ); pigs received a control diet and were injected with 0·9 % sterile saline); LPS-challenged control (LPSCC (
); pigs received a control diet and were injected with 0·9 % sterile saline); LPS-challenged control (LPSCC (![]() ); pigs received the same control diet and were challenged with LPS); LPS+0·5 % Asn (
); pigs received the same control diet and were challenged with LPS); LPS+0·5 % Asn (![]() , pigs received a 0·5 % Asn diet and were challenged with LPS); LPS+1·0 % Asn (
, pigs received a 0·5 % Asn diet and were challenged with LPS); LPS+1·0 % Asn (![]() , pigs received a 1·0 % Asn diet and were challenged with LPS). LPSCC v. CONTR was used to determine the response to LPS challenge. Linear (L) and quadratic (Q) polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged piglets. There was treatment × time interaction for protein expression of liver HSP70 (P< 0·001). At 4 h: LPSCC v. CONTR (P= 0·337), L (P= 0·275), Q (P= 0·098) and at 24 h: LPSCC v. CONTR (P= 0·291), L (P= 0·001), Q (P= 0·006).
, pigs received a 1·0 % Asn diet and were challenged with LPS). LPSCC v. CONTR was used to determine the response to LPS challenge. Linear (L) and quadratic (Q) polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged piglets. There was treatment × time interaction for protein expression of liver HSP70 (P< 0·001). At 4 h: LPSCC v. CONTR (P= 0·337), L (P= 0·275), Q (P= 0·098) and at 24 h: LPSCC v. CONTR (P= 0·291), L (P= 0·001), Q (P= 0·006).
At 24 h, TNF-α protein concentration was undetectable except for the CONTR group. Relative to the CONTR piglets, LPS challenge decreased liver TNF-α mRNA abundance (P< 0·01), and tended to decrease liver HSP70 mRNA abundance (P= 0·099). With increasing Asn supplementation, liver TNF-α mRNA abundance and HSP70 protein abundance were found increased (linear, P< 0·01; quadratic, P< 0·05) in LPS-challenged pigs.
Liver total nitric oxide synthase and inducible nitric oxide synthase activities
No significant treatment × time interactions were observed for liver tNOS and iNOS activities (Table 3). Overall, relative to the CONTR piglets, LPS challenge decreased liver iNOS activity (P< 0·05). With increasing Asn supplementation, liver iNOS activity was found increased (linear, P< 0·05; quadratic, P= 0·054) in LPS-challenged pigs. Neither LPS nor Asn supplementation had any effect on liver tNOS activity.
Table 3 Effects of asparagine (Asn) supplementation on liver nitric oxide synthase (NOS) activity after 4 or 24 h Escherichia coli lipopolysaccharide (LPS) challenge in weanling piglets (Mean values with their pooled standard errors, n 6)
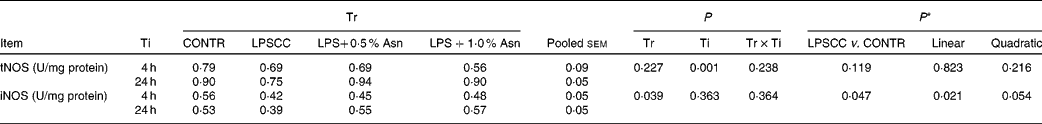
Tr, Treatment; Ti, Time; CONTR, non-challenged control (piglets received a control diet and were injected with 0·9 % sterile saline); LPSCC, LPS-challenged control (piglets received the same control diet and were injected with Escherichia coli LPS); LPS+0·5 % Asn, piglets received a 0·5 % Asn diet and were injected with LPS; LPS+1·0 % Asn, piglets received a 1·0 % Asn diet and were injected with LPS; tNOS, total NOS; iNOS, inducible NOS.
* LPSCC v. CONTR was used to determine the response to LPS challenge. Linear and quadratic polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged piglets.
Liver mRNA expression of Toll-like receptor 4 and nucleotide-binding oligomerisation domain protein signalling and their downstream signals
Significant treatment × time interactions were found for mRNA abundance of liver TLR4, myeloid differentiation factor 88 (MyD88), IL-1 receptor-associated kinase 1 (IRAK1), TNF-α receptor-associated factor 6 (TRAF6), NOD1, NOD2, receptor-interacting serine/threonine-protein kinase 2 (RIPK2) and NF-κB p65 (P< 0·01; Table 4). At 4 h, relative to the CONTR piglets, LPS challenge increased mRNA abundance of liver TLR4, MyD88, IRAK1, NOD1, NOD2, RIPK2 and NF-κB p65 (P< 0·01), and tended to increase mRNA abundance of liver TRAF6 (P= 0·056). With increasing Asn supplementation, mRNA abundance of liver TLR4, IRAK1, TRAF6, NOD1, NOD2 and NF-κB p65 were found decreased (linear and quadratic, P< 0·05), and RIPK2 tended to be decreased (quadratic, P= 0·088) in LPS-challenged pigs.
Table 4 Effects of asparagine (Asn) supplementation on mRNA abundance of Toll-like receptor 4 (TLR4) and nucleotide-binding oligomerisation domain protein (NOD) and their downstream signalling molecules after 4 or 24 h Escherichia coli lipopolysaccharide (LPS) challenge in weanling piglets (Mean values with their pooled standard errors, n 6)
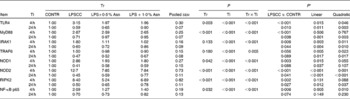
Tr, Treatment; Ti, Time; CONTR, non-challenged control (piglets received a control diet and were injected with 0·9 % sterile saline); LPSCC, LPS-challenged control (piglets received the same control diet and were injected with Escherichia coli LPS); LPS+0·5 % Asn, piglets received a 0·5 % Asn diet and were injected with LPS; LPS+1·0 % Asn, piglets received a 1·0 % Asn diet and were injected with LPS; TLR4, Toll-like receptor 4; MyD88, myeloid differentiation factor 88; IRAK1, IL-1 receptor-associated kinase 1; TRAF6, TNF-α receptor-associated factor 6; RIPK2, receptor-interacting serine/threonine-protein kinase 2.
* LPSCC v. CONTR was used to determine the response to LPS challenge. Linear and quadratic polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged piglets.
At 24 h, relative to the CONTR piglets, LPS challenge decreased mRNA abundance of liver TLR4, MyD88, IRAK1, NOD2 and RIPK2 (P< 0·05), and tended to decrease mRNA abundance of liver TRAF6 (P= 0·089), NOD1 (P= 0·088) and NF-κB p65 (P= 0·074). With increasing Asn supplementation, mRNA abundance of liver TLR4, MyD88, IRAK1, TRAF6, NOD2 and RIPK2 were found increased linearly and quadratically (P< 0·05), and NOD1 was found increased linearly (P< 0·05) in LPS-challenged pigs.
Liver mRNA expression of negative regulators of Toll-like receptor 4 and nucleotide-binding oligomerisation domain protein signalling pathways
Significant treatment × time interactions were found for mRNA abundance of liver radioprotective 105 (RP105), suppressor of cytokine signalling 1 (SOCS1), single Ig IL-1R-related molecule (SIGIRR) (P< 0·01), Erbb2 interacting protein (ERBB2IP) (P= 0·056) and centaurin β1 (CENTB1) (P= 0·062) (Table 5). At 4 h, compared to the CONTR piglets, LPS challenge increased mRNA abundance of liver SOCS1, and decreased mRNA abundance of liver SIGIRR and CENTB1 (P< 0·05).
Table 5 Effects of asparagine (Asn) supplementation on liver mRNA abundance of negative regulators of Toll-like receptor 4 (TLR4) and nucleotide-binding oligomerisation domain protein (NOD) signal pathway after 4 or 24 h Escherichia coli LPS challenge in weanling piglets (Mean values with their pooled standard errors, n 6)
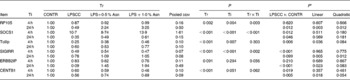
Tr, Treatment; Ti, Time; CONTR, non-challenged control (piglets received a control diet and were injected with 0·9 % sterile saline); LPSCC, LPS-challenged control (piglets received the same control diet and were injected with Escherichia coli LPS); LPS+0·5 % Asn, piglets received a 0·5 % Asn diet and were injected with LPS; LPS+1·0 % Asn, piglets received a 1·0 % Asn diet and were injected with LPS; RP105, radioprotective 105; SOCS1, suppressor of cytokine signalling 1; Tollip, Toll-interacting protein; SIGIRR, single Ig IL-1R-related molecule; ERBB2IP, Erbb2 interacting protein; CENTB1, centaurin β1.
* LPSCC v. CONTR was used to determine the response to LPS challenge. Linear and quadratic polynomial contrasts were used to determine the response to Asn supplementation among LPS-challenged piglets.
At 24 h, relative to the CONTR piglets, LPS challenge decreased mRNA abundance of liver RP105, SOCS1, ERBB2IP and CENTB1 (P< 0·05), and tended to decrease mRNA abundance of liver SIGIRR (P= 0·099). With increasing Asn supplementation, mRNA abundance of liver RP105 (linear, P< 0·01; quadratic, P< 0·05), SIGIRR (linear, P< 0·01; quadratic, P< 0·05), ERBB2IP (linear, P< 0·05; quadratic, P= 0·083) and CENTB1 (linear, P< 0·05; quadratic, P= 0·054) were found increased, and SOCS1 tended to be increased (linear, P= 0·069) in LPS-challenged pigs.
No significant treatment × time interaction was observed for liver Toll-interacting protein (Tollip) mRNA abundance. Overall, compared to the CONTR pigs, LPS challenge decreased liver Tollip mRNA abundance (P< 0·01). With increasing Asn supplementation, mRNA abundance of liver Tollip was found increased (linear, P< 0·05; quadratic, P= 0·084) in LPS-challenged pigs.
Discussion
The intraperitoneal injection of LPS is widely used for the study of liver injury( Reference Chen, Liu and Zhu 3 , Reference Schmöcker, Weylandt and Kahlke 4 , Reference Li, Liu and Che 25 ). As the integral component of the cell wall of Gram-negative bacteria, LPS is prototypical example of endotoxin( Reference Schmöcker, Weylandt and Kahlke 4 , Reference Koenderman, Buurman and Daha 27 ). Substantial evidence has shown that LPS stimulates Kupffer cells to secrete pro-inflammatory cytokines, especially TNF-α, the critical mediator of liver damage( Reference Bradham, Plümpe and Manns 5 ). Our previous research showed that dietary supplementation of 0·5 and 1·0 % Asn alleviated growth suppression of weaned piglets after LPS challenge( Reference Li, Liu and Shi 24 ). So, in the present study, we took advantage of the model for inducing liver injury by injecting LPS, to investigate whether 0·5 and 1·0 % Asn supplementation could alleviate liver injury in weaned pigs.
In clinical practice, various biochemical tests are used to index liver injury, such as blood ALT, AST, GGT and AKP activities, and ALT:AST ratio( Reference Lee and Yang 28 , Reference Van Beek, de Moor and de Geus 29 ). In the present study, with increasing Asn supplementation, serum AST and AKP activities were found decreased linearly and quadratically in LPS-challenged pigs at 24 h post-injection. Asn also increased serum ALT activity and ALT:AST ratio linearly and quadratically. These results were also supported by histological observations; Asn alleviated severe hepatic injury such as hepatocyte caryolysis, karyopycnosis and inflammatory cell infiltration caused by LPS challenge. Therefore, these results indicate that Asn supplementation attenuated the damage of hepatic architecture and function caused by LPS challenge. Until now, research related to Asn modulation of liver health was very limited. We speculate that the protective effect of Asn on liver might be due to the following mechanisms. Asn can be readily converted into aspartate in response to immunological challenges. Aspartate enters the urea cycle, and the amino group from aspartate is required for the recycling of the citrulline into arginine in activated macrophages( Reference Li, Yin and Li 15 , Reference Adam, Yang and Bauerschmidt 30 ). Many studies have shown that arginines maintain the hepatic integrity, and exert a protective effect in many liver injury models( Reference Li, Liu and Che 25 , Reference Wu, Bazer and Davis 31 ). The carbon skeleton from aspartate leaves the urea cycle as fumarate which can participate in the citric acid cycle, and it increases cellular energy production( Reference Adam, Yang and Bauerschmidt 30 ). In addition, aspartate is important for other aminotransferase reactions, purine and pyrimidine nucleotides synthesis, and shuttling between cytosol and the mitochondrion for gluconeogenesis, besides a role for the urea cycle( Reference Li, Yin and Li 15 ).
The tight junction is a pivotal cellular component for maintenance of tissue integrity, and it forms the continuous intercellular barrier against pathogen invasion. Deregulation and functional abnormality of tight junctions result in the development of liver disease( Reference Lee and Luk 32 , Reference Anderson and Van Itallie 33 ). Claudins are considered as the main constituents of the tight junction and important regulators of paracellular permeability. Consistent with the improved hepatic architecture and function, liver claudin-1 protein expression was found increased linearly and quadratically with increasing Asn supplementation at 24 h after LPS challenge, which suggests that Asn may improve liver integrity partially via improving the expression of liver tight junction proteins.
We hypothesised that Asn enhanced hepatic integrity by regulating hepatic inflammatory response. Increased level of TNF-α is an important marker of inflammation( Reference Chen, Liu and Zhu 3 , Reference Jacobi, Moeser and Corl 34 ). Several instances of evidence have demonstrated that overproduction of pro-inflammatory mediators (especially TNF-α) results in liver injury( Reference Chen, Liu and Zhu 3 , Reference Kanuri, Spruss and Wagnerberger 35 ). HSP have mostly been regarded as intracellular molecules that mediate a range of essential housekeeping and cytoprotective functions( Reference Shi, Dong and Wei 36 ). High level of intracellular HSP70 can reduce the inflammatory response and improve liver regeneration( Reference Oka, Akagi and Kinugasa 37 ). Intracellular HSP70 has been shown to directly interact with the transcription factor NF-κB, and prevent its activation( Reference Kuboki, Schuster and Blanchard 38 ). In our present study, at 4 h after LPS challenge, consistent with histological and biochemical alterations in liver, LPS increased hepatic TNF-α mRNA and protein abundances and HSP70 mRNA abundance. It is possible that, LPS increased the expression of pro-inflammatory mediators (e.g. TNF-α), which further induced the expression of anti-inflammatory mediators (e.g. intracellular HSP70) by a negative feedback. With increasing Asn supplementation, mRNA abundances of TNF-α and HSP70 were decreased linearly and quadratically, and protein abundance of HSP70 tended to be decreased linearly and quadratically among the LPS-challenged pigs. Surprisingly, further ELISA analysis showed that liver TNF-α protein concentration was not affected by Asn supplementation at 4 h after LPS challenge. A recent research investigating the correlation of mRNA and protein expressions has shown that mRNA and protein are differentially expressed( Reference Anderson and Seilhamer 39 ). These results indicate that, in early stage of LPS challenge, Asn might attenuate hepatic injury by decreasing the mRNA expression of pro-inflammatory mediators.
However, interestingly, at 24 h after LPS challenge, LPS challenge reduced mRNA expression of hepatic TNF-α, and TNF-α mRNA expression was found increased linearly and quadratically with increasing Asn supplementation in the LPS-challenged pigs. Several studies have shown that Kupffer cells also have the potential to exert stimulatory influences on hepatocyte regeneration by producing many kinds of cytokines, such as IL-6 and TNF-α( Reference Malik, Selden and Hodgson 40 , Reference Yokoyama, Nagino and Nimura 41 ). Bradham et al. ( Reference Bradham, Plümpe and Manns 5 ) reported that a low expression of TNF-α is not beneficial, especially for hepatic regeneration. In addition, Akerman et al. ( Reference Akerman, Cote and Yang 42 ) reported that hepatocyte DNA synthesis was inhibited after partial hepatectomy in rats pretreated with TNF-α antibody. Moreover, Yang et al. ( Reference Yang, Zou and Koskinen 43 ) reported that some inflammatory factors might contribute to hepatic damage in early stage of liver injury, but could enhance liver regeneration in late stage, and prolonged anti-inflammatory treatment in late stage was not beneficial for hepatocyte regeneration. Mounting evidence suggests that HSP70 is also released from stressed cells as an extracellular protein that can serve as a paracrine signal. Extracellular HSP70 are capable of inducing the expression of inflammatory cytokines IL-6 and TNF-α, both of which are considered integral to liver regeneration( Reference Wolf, Bhatti and Fouraschen 44 ). Shi et al. ( Reference Shi, Dong and Wei 36 ) found that hepatic protein expression of HSP70 increased at the early phase of liver regeneration (24 h after partial hepatectomy), and declined to the constitutively low level later, which indicated that HSP70 played an important role in liver regeneration. In our present study, at 24 h after LPS challenge, liver HSP70 protein expression was found increased linearly and quadratically with increasing Asn supplementation in the LPS-challenged pigs. These results indicate that, in late stage of LPS challenge, Asn might improve hepatocyte regeneration, by increasing the mRNA expression of TNF-α and the protein expression of liver HSP70. Collectively, in early and late stages of LPS challenge, Asn exerts different regulatory effects on the protein expression of hepatic HSP70 and mRNA expression of TNF-α, and improves liver integrity.
In animal tissues, NO is generated enzymatically by synthases (NOS), which oxidises l-arginine to l-citrulline( Reference Wu, Bazer and Davis 31 ). There are three isoforms of NOS. Of them, iNOS is present in various cell types upon pro-inflammatory cytokine stimulation. In macrophages, monocytes and other cells, the induction of iNOS and the presence of l-arginine are sufficient to initiate the generation of NO, which have been shown to facilitate the process of hepatic regeneration( Reference Yokoyama, Nagino and Nimura 41 , Reference Guzik, Korbut and Adamek-Guzik 45 , Reference Malyshev, Bayda and Trifonov 46 ). In our present study, LPS reduced iNOS activities, and iNOS activity was found increased linearly and quadratically with increasing Asn supplementation in the LPS-challenged pigs. These results indicate that Asn may improve liver regeneration partially via improving iNOS activity.
To elucidate the molecular mechanism(s) by which Asn modulated hepatic inflammatory response, we investigated the role of two inflammatory signalling pathways, including transmembrane TLR and intracellular NOD( Reference Al-Sayeqh, Loughlin and Dillon 47 ). Among the TLR family, TLR4 is the most studied member which has been detected on all types of liver cells including Kupffer cells( Reference Szabo, Dolganiuc and Mandrekar 8 , Reference Becker and O'Neill 48 ). When TLR4 binds its ligand LPS, the TLR4/myeloid differentiation protein 2/cluster of differentiation 14 receptor complex transduces a signal sensed by MyD88, which recruits IRAK to activate TRAF6, and then further activates the IκB kinase (IKK) complex. The activated IKK phosphorylates the IκB family, and leads ultimately to the activation of NF-κB( Reference Becker and O'Neill 48 ). Among the NOD family, NOD1 and NOD2 are the best-characterised members, and are also highly expressed in liver cells. Similar to TLR4, NOD1 and NOD2 can also activate NF-κB via their adaptor molecule, RIPK2. Activation of NF-κB results in expression of the inflammatory genes, such as pro-inflammatory cytokines. In the present experiment, consistent with the changes of mRNA abundance of pro-inflammatory mediators, we also observed that hepatic mRNA abundances of TLR4 signalling-related genes (TLR4, MyD88, IRAK1 and TRAF6), NOD signalling-related genes (NOD1, NOD2 and RIPK2) and NF-κB p65 were found decreased linearly or quadratically at 4 h post-injection; they were found increased linearly or quadratically at 24 h post-injection, with increasing Asn supplementation in the LPS-challenged pigs. So, it is possible that the beneficial effects of Asn on hepatic integrity were closely related to down-regulating mRNA expressions of TLR4 and NOD signalling-related genes in early stage of LPS challenge, and up-regulating mRNA expressions of TLR4 and NOD signalling-related genes in late stage.
Activation of TLR4 and NOD signalling triggers the production of pro-inflammatory cytokines and inflammatory response, which is crucial for host-defensive responses against invading pathogens. However, the aberrant activation of TLR4 and NOD signalling also can elicit collateral host-tissue injury( Reference Kondo, Kawai and Akira 49 , Reference Coll and O'Neill 50 ). To prevent excessive and harmful inflammatory responses, TLR4 and NOD signalling are negatively controlled by multiple mechanisms. Among them, negative regulators of TLR4 and NOD signalling play an important role in this process( Reference Coll and O'Neill 50 ). To date, many negative regulators have been identified and characterised( Reference Kondo, Kawai and Akira 49 , Reference Coll and O'Neill 50 ). Of them, Tollip, RP105, SOCS1 and SIGIRR are typical negative regulators of TLR4 signalling, and ERBB2IP and CENTB1 are typical negative regulators of NOD signalling.
In our present study, at 4 h post-injection, LPS challenge decreased mRNA abundances of liver SIGIRR and CENTB1, and increased mRNA abundance of liver SOCS1. Similarly, Ueno-Shuto et al. ( Reference Ueno-Shuto, Kato and Tasaki 51 ) reported that LPS can decrease SIGIRR expression via TLR4-p38 pathway in non-epithelial innate immune cells. In addition, Fujimoto & Naka( Reference Fujimoto and Naka 52 ) reported that excessive stimulation with cytokines or TLR ligands increased the expression of SOCS1 in liver, and SOCS1 expression in the liver prevented fatal hepatitis via the suppression of exacerbated liver inflammation. These results indicate that, in early phase of LPS challenge, loss of some negative regulators (SIGIRR and CENTB1) of TLR and NOD led to hyper-activation of TLR signalling. To maintain homeostasis, other negative regulators (SOCS1) might be increased to suppress overactivation of inflammatory responses( Reference Kondo, Kawai and Akira 49 ).
At 24 h post-injection, LPS decreased mRNA abundances of liver RP105, SOCS1, ERBB2IP and CENTB1, which is consistent with the decreased liver mRNA abundances of TLR4 and NOD signalling-related genes. It is possible that, in late stage of LPS challenge, the decreased mRNA expressions of TLR4 and NOD negative regulators is due to low activation of TLR4 and NOD signalling pathways in LPS-challenged pigs. With increasing Asn supplementation, mRNA expressions of liver RP105, SIGIRR, ERBB2IP and CENTB1 were up-regulated linearly and quadratically, which is consistent with the up-regulated liver mRNA expressions of TLR4 and NOD signalling-related genes at 24 h post-injection. It is possible that, in LPS-challenged pig fed the Asn diet, the increased mRNA expressions of TLR4 and NOD signalling-related genes induced the increased mRNA expressions of their negative regulators in a negative feedback.
In addition, with increasing Asn supplementation, mRNA expression of liver Tollip was found increased linearly and quadratically in the LPS-challenged pigs. High level of Tollip can suppress overactivation of inflammatory response caused by TLR4 signalling( Reference Steenholdt, Andresen and Pedersen 53 ). So, it is possible that Asn attenuated hepatic injury partially via improving the mRNA expression of Tollip.
In our present study, dynamic changes occur in pro-inflammatory gene expression in liver after LPS challenge( Reference Xu, You and Li 54 ). So, measurements taken only in early and late stages (4 and 24 h) are probably not adequate. In addition, only two levels of Asn supplemented (0·5 and 1·0 % Asn) were used in our present study. Compared with lower concentration of Asn (0·5 %), higher concentration (1·0 %) had better effect on liver morphology, serum biochemical parameters, claudin-1 and HSP70 protein expressions, mRNA abundance of hepatic TLR4 and NOD signalling-related genes and their negative regulators, especially at 24 h after LPS challenge, but it had almost the same effect on other data in our present study. So, 1·0 % Asn supplement might not be the most appropriate dose. If we had had more doses of Asn, the most appropriate doses would have been obtained for improving liver integrity, for the quantification of which further experiments are necessitated. Moreover, measurements only for mRNA abundances of TLR and NOD signalling-related molecules (for example, TLR4, NOD and NF-κB p65 mRNA expressions) are not sufficient to confirm the roles of these signalling molecules in liver injury. Thus, in future studies, further measurements such as of TLR4 or NOD activation and NF-κB nuclear translocation at more time points are also needed to understand better their roles in LPS-induced liver injury.
In conclusion, Asn supplementation improves liver integrity in LPS-challenged piglets. In addition, Asn inhibits the expressions of hepatic pro-inflammatory mediators, and TLR4 and NOD signalling-related genes in early stage of LPS challenge, and increases NOS activity, HSP70 synthesis and the expressions of hepatic pro-inflammatory mediators, TLR4 and NOD signalling-related genes and their negative regulators in late stage.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114515001476
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (31422053, 31372318 and 31172222) and the Project of Natural Science Foundation of Hubei Province (2013CFA029).
The authors' contributions are as follows: Y. L. contributed to the study design, participated in the statistical analysis and interpretation of data, and writing of the paper; H. W. collected data, participated in the statistical analysis and interpretation of data, and writing of the paper; D. P. and W. L. collected data and participated in the statistical analysis; H. Z. contributed to the study design, participated in the statistical analysis and interpretation of data; Y. H., S. L., H. S. and X. W. contributed to data collection; Y. L. had primary responsibility for the final content. All authors read and approved the final version of the manuscript.
The authors declare that they have no conflict of interest.














