At present, childhood obesity represents a challenge for healthcare providers as it has been widely shown that general and abdominal fat are significantly associated with the development of ischaemic cardiac events( Reference Yarnell, Patterson and Thomas 1 ), type 2 diabetes mellitus in adults( Reference Schulze, Heidemann and Schienkiewitz 2 ) and risk prediction of cardiometabolic disease in children and adolescents( Reference Freedman, Kahn and Mei 3 ). Obesity is considered as a state of sub-clinical inflammation characterised by the secretion of cytokines that promote atherosclerotic plaque formation and consequent endothelial dysfunction( Reference Lyon, Law and Hsue 4 ). Consequently, it has been necessary to develop diagnostic tools for early detection of obesity in the paediatric population as a public health prevention strategy. BMI and waist circumference (WC) have been widely used as measures to evaluate the impact of obesity on the risk for cardiometabolic diseases in children( Reference Mokha, Srinivasan and DasMahapatra 5 ).
There has been a growing interest in the waist:height ratio (W-HtR) as an adiposity index, with two recent meta-analyses showing that W-HtR has a greater predictive ability of cardiovascular and metabolic risk than classic anthropometric indicators( Reference Savva, Lamnisos and Kafatos 6 , Reference Ashwell, Gunn and Gibson 7 ). A W-HtR≥0·50 has been proposed as the cut-off point for predicting the risk for CVD and diabetes( Reference Browning, Hsieh and Ashwell 8 ). In addition, some authors state that W-HtR can be the most useful clinical tool for the global detection of abdominal obesity and cardiometabolic risk screening in children and adults( Reference Ashwell and Hsieh 9 ). However, very few large studies have evaluated the association between W-HtR and cardiometabolic risk in children and adolescents in Latin America. Moreover, multivariate analyses verifying whether this association is independent of located subcutaneous fat have not been conducted so far. The aim of the present study was to evaluate the association of W-HtR and WC with metabolic syndrome (MetS) abnormalities and high LDL-cholesterol in schooled adolescents by considering adjustments for trunk skinfolds and also BMI.
Methods
The study’s sample consisted of individuals from a cross-sectional study, the IFRECNTEC Study (identification of risk factors for adult non-communicable chronic disease in schooled population), conducted in Cali, Colombia( Reference Gracia, de Plata and Méndez 10 ). The IFRECNTEC Study evaluated a sample of 2807 subjects aged 5–18 years. Our analysis excluded children aged <10 years (n 628), adult individuals (≥18 years, n 91) and cases with missing values for the study variables (n 416). The final remaining sample consisted of 1672 adolescents. Informed written consent was obtained from both the parent and the child. The study was reviewed and approved by the Ethics Committee at Universidad del Valle.
Blood was collected via venepuncture after an overnight fast. Concentrations of fasting glucose, total cholesterol, HDL-cholesterol and TAG were determined using enzymatic-colorimetric assays. LDL-cholesterol level was estimated using the Friedwald equation( Reference Friedewald, Levy and Fredrickson 11 ). Blood pressure was measured using mercury sphygmomanometers with an appropriately sized cuff in a sitting position after 15 min of rest. Phase I and V (disappearance) Korotkoff sounds were used to identify systolic blood pressure (SBP) and diastolic blood pressure (DBP)( Reference Franklin, Gustin and Wong 12 ). Body weight and height were measured using standard techniques and instruments( Reference Lohman, Roche and Martorell 13 ). BMI was calculated as weight/height2 ( Reference Keys, Fidanza and Karvonen 14 ). WC was measured from the midpoint between the lateral iliac crest and the lowest rib using a flexible steel tape measure( Reference Lohman 15 ). W-HtR was calculated as WC divided by height. The subscapular skinfold (SS), abdominal skinfold (AS) and supra-iliac skinfold (SIS) were measured using skinfold calipers in the specific locations( Reference Lohman, Roche and Martorell 13 ).
Metabolic syndrome abnormalities and high LDL-cholesterol
We used the abnormalities of MetS according to the criteria by Cook et al. for paediatrics as follows( Reference Cook, Weitzman and Auinger 16 , Reference Costa, Santos and Goldraich 17 ): high levels of TAG as levels ≥110 mmol/l, low HDL-cholesterol as levels ≤40 mmol/l and high blood pressure as SBP and/or DBP at or above the 90th percentile for age, sex and height according to the National Heart, Lung, and Blood Institute tables( 18 ). However, for high fasting glucose, we avoided using Cook et al.’s cut-off point of glucose as ≥110 mmol/l, as even the current adult guidelines recommend lower cut-off points. Therefore, we adopted the criterion from the International Diabetes Federation definition, which suggests high glucose levels as values ≥100 mmol/l( Reference Zimmet, Alberti and Kaufman 19 ). In addition, high levels of LDL-cholesterol were also studied and defined as values ≥130 mmol/l( Reference Kit, Carroll and Lacher 20 ). A clustering of two or more of the variables mentioned above was similarly evaluated.
Data analyses
As most of the variables presented an abnormal distribution, medians and their interquartile ranges were used for the description of the study population by sex, and difference between these groups was estimated via the Mann–Whitney U test. Values of WC and W-HtR were evaluated as age- and sex-specific quartiles. In each sex, Poisson regressions were performed to assess associations of highest quartiles of WC and W-HtR with MetS components and high LDL-cholesterol and with the fact of having two or more of these abnormalities. Each association was adjusted for the highest quartiles (also age- and sex-specific) of each trunk skinfold and BMI in separated models. Additional adjustment for age (continuous) was also evaluated. However, this covariate did not modify the effect estimates from unadjusted and adjusted models, presumably due to stratification by age in quartiles of WC, W-HtR, trunk skinfolds and BMI. All the analyses were processed using STATA 8.0 software (Statistics/Data Analysis, Stata Corp.).
Results
Study variables are described by sex in Table 1. Boys had higher values of WC and W-HtR, as well as higher prevalence of high fasting glucose and blood pressure. The prevalence of high levels of TAG and values of all the skinfold thickness measurements were higher in girls. There were no differences by sex regarding the prevalence of the cluster of abnormalities (≥2) or overweight/obesity. An additional description of the study variables by age groups is shown in online Supplementary Table S1. Overall, the number of male and female adolescents in each age group was comparable. All the biochemical variables tended to decrease across age groups, whereas blood pressure and all the anthropometrical variables tended to increase with age.
Table 1 Description of the study population (Medians and their interquartile ranges, number of subjects and percentages)

WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure.
The highest quartile of WC (v. the other quartiles) was positively associated with high levels of TAG in all the adjustment models for both sexes (Table 2), and with high blood pressure in female adolescents. In girls, having at least two abnormalities was significantly associated with high WC before and after adjusting for each trunk’s skinfold but not when adjusting for BMI. In boys, significant associations between high WC and high fasting glucose and low HDL-cholesterol were no longer significant in most of the adjustments for adiposity markers (Table 2). In girls, a significant association between high WC and low HDL-cholesterol was attenuated when SS or BMI scores were covariates in the regression models. Unexpectedly high WC became inversely associated with high fasting glucose in the SS-adjusted model and with high LDL-cholesterol in the BMI-adjusted model (Table 2).
Table 2 Prevalence ratios for metabolic syndrome components and high LDL-cholesterol by age- and sex-specific quartiles (Q) of waist circumference (highest quartile v. quartiles 1–3) in the adolescents(Prevalence ratios and 95%confidence intervals)
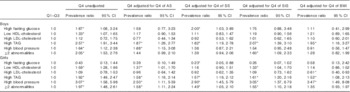
AS, abdominal skinfold; SS, subscapular skinfold; SIS, supra-iliac skinfold.
* Significant associations (P<0·05).
The highest quartile of W-HtR (v. the other quartiles) was positively and significantly associated with high levels of TAG, despite adjustments for trunk’s skinfolds and BMI in girls, whereas in boys this association was attenuated after adjustments, except in the SIS-adjusted model (Table 3). In both sexes, a significant relationship between having at least two abnormalities and high W-HtR was lost after adjusting for SS, SIS or BMI. In girls, an initial significant association between high W-HtR and low HDL-cholesterol did not remain significant after further adjustment for other adiposity markers (Table 3).
Table 3 Prevalence ratios for metabolic syndrome components and high LDL-cholesterol by age- and sex-specific quartiles (Q) of waist:height ratio (highest quartile v. quartiles 1–3) in adolescents(Prevalence ratios and 95%confidence intervals)
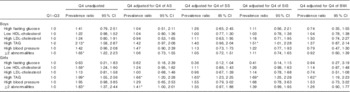
AS, abdominal skinfold; SS, subscapular skinfold; SIS, supra-iliac skinfold.
* Significant associations (P<0·05).
Discussion
In the present study, we evaluated the association between WC and W-HtR and cardiovascular risk factors. In addition, we assessed whether these associations were independent of located subcutaneous adiposity and BMI. High WC values were found to be consistently and independently associated with high TAG levels in both sexes, and with high blood pressure specifically in female adolescents. The W-HtR was scarcely associated with MetS components, although it was independently associated with high TAG levels in female adolescents.
WC – but not W-HtR – was significantly and independently associated with high blood pressure in our study, and previous studies have presented conflicting results. Mirmiran et al.( Reference Mirmiran, Rezaei and Asghari 21 ) reached similar conclusions in 134 Iranian adolescents (10–18 years old). Moreover, two studies with Mexican and Dutch children reported associations with both adiposity markers, but WC was better associated with high blood pressure than W-HtR( Reference Balas-Nakash, Villanueva-Quintana and Tawil-Dayan 22 , Reference Sijtsma, Bocca and L’Abée 23 ). In addition, W-HtR was deemed unsuitable as a screening tool for high blood pressure in German adolescents aged 11–17 years( Reference Kromeyer-Hauschild, Neuhauser and Schaffrath Rosario 24 ), and Bailey et al.( Reference Bailey, Savory and Denton 25 ) did not find any association between WC and W-HtR and SBP in 234 British children aged 10–19 years. Likewise, these associations have also been inconclusive in adults. For instance, although Li et al.( Reference Li, Chen and Chang 26 ) and Park et al.( Reference Park, Choi and Lee 27 ) found a stronger association of blood pressure with W-HtR than with WC in Asian adult populations, Valenzuela and Bustos reported comparable associations with both adiposity markers in young Chilean adults( Reference Valenzuela and Bustos 28 ).
High WC values were found to be consistently and independently associated with high levels of TAG in both sexes. This finding is in line with studies by Sijtsma et al.( Reference Sijtsma, Bocca and L’Abée 23 ) and Harrington et al.( Reference Harrington, Staiano and Broyles 29 ), who reported significant positive associations between WC and TAG in pre-pubertal and pubertal children. This finding was also related to WC as a good predictive factor of hypertriglyceridaemia in a sample of 41 087 adults( Reference Du, Ma and Li 30 ). In this study, we have extended the knowledge about this relationship by finding it to be independent of other adiposity markers.
Previous studies have reported associations between high levels of TAG and W-HtR in paediatric populations. Graves et al.( Reference Graves, Garnett and Cowell 31 ) reported a prospective association between W-HtR in childhood and cardiometabolic outcomes in adolescents, with an increased risk in boys. A cross-sectional study by Balas-Nakash et al.( Reference Balas-Nakash, Villanueva-Quintana and Tawil-Dayan 22 ) describes a similar finding, adjusting for age, sex, physical activity, hours per week of television, video games or computer, energy consumption and percentage of dietary lipids. In contrast, this association was NS in an unadjusted analysis carried out by Feliciano Pereira et al.( Reference Feliciano Pereira, Serrano and Queiroz Carvalho 32 ), and in another study WC was found to be more strongly related to the levels of TAG than W-HtR( Reference Mirmiran, Rezaei and Asghari 21 ), adjusting for sex and physical activity. Our study, with regard to the relationship between W-HtR and high TAG levels, is in line with most of the studies mentioned above based on associations not adjusted for other adiposity markers. However, a sex-specific relationship was evident, as only in the female adolescents the association remained significant, despite adjustment for skinfolds or BMI.
High LDL-cholesterol was the abnormality less associated with WC and W-HtR in the adolescents from our study, even in unadjusted associations. With regard to this relationship, there are conflicting results in previous studies as well. In pre-pubertal children, Sijtsma et al. did not find correlations between WC, W-HtR and LDL-cholesterol, whereas Hara et al.( Reference Hara, Saitou and Iwata 33 ) reported significant association in boys and girls aged 9–13 years. Recently, White and Jago( Reference White and Jago 34 ) found a strong and significant association between the change in WC and the change in LDL-cholesterol levels in a cohort of 617 female adolescents during the 10 years of follow-up. On the other hand, LDL-cholesterol was the risk marker less associated with WC in a large epidemiological study in adults( Reference Bennasar-Veny, Lopez-Gonzalez and Tauler 35 ).
The unexpected inverse associations of WC with high fasting glucose levels in the SS-adjusted model and with high LDL-cholesterol in the BMI-adjusted model could be chance findings. This is due to the fact that these associations were not consistently significant throughout the rest of the adjustment models. Alternatively, it is also possible that some confounder not evaluated in this study might have had an influence on these findings.
Despite adjustments for other adiposity markers attenuating some relationships, our general finding is that WC itself is a better indicator of cardiometabolic risk than W-HtR, especially based on vascular and lipid risk factors. In other words, our results do not support W-HtR as a relevant adiposity marker related to cardiometabolic risk in adolescence. This agrees with a body of literature in young children and adults in which W-HtR was not found to be better associated with cardiometabolic risk factors or MetS than WC( Reference Sijtsma, Bocca and L’Abée 23 , Reference Bener, Yousafzai and Darwish 36 ). In addition, the conflicting reports from studies mentioned above regarding associations with blood pressure and LDL-cholesterol could be explained by differences in statistical power, methodological approaches (e.g. adjustments, laboratory tests) and populations (e.g. young children/adolescents, ethnicity). Multicentre studies are warranted for a better interpretation of the associations between WC, W-HtR and cardiometabolic risk markers.
Data on pubertal changes, ethnicity and physical activity were not available for this analysis and these variables would have allowed for more robust adjustments. However, the age variable is strongly related to pubertal changes, and the stratification by age that we performed for WC and W-HtR may have decreased the bias of not adjusting for pubertal development. Further, adjustments for each trunk’s skinfolds in the present study allowed for obtaining a more precise independence profile for the evaluated associations, an approach that had not been considered in previous studies.
In conclusion, the findings of this study suggest that abdominal adiposity itself would be a better indicator of cardiometabolic risk in adolescents than abdominal adiposity regarding height. This observation does not support W-HtR as a relevant adiposity marker for cardiovascular and metabolic risks in adolescence.
Acknowledgements
The authors thank Dr Daniel Herrera-Kelly and Dr Paul Kawale for their support on English language grammar and style.
This work was funded by a grant from the Colombian Administrative Department for Development and Science Technology (COLCIENCIAS).
R. A. A. Z. conceived the research question and wrote the manuscript. C. A. d. P. coordinated field work, researched data and edited the manuscript. M. F. S. O. conceived the study design, analysed the data and wrote the manuscript.
The authors declare no conflicts of interest.
Supplementary Material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S0007114515002275








