INTRODUCTION
Acute respiratory infections (ARI) remain the leading cause of disease and death in young children in low- and middle-income countries, accounting for almost 1·4 million annual deaths, i.e. almost a fifth of all deaths in children aged <5 years worldwide [Reference Liu1]. Over 90% of these pneumonia-associated deaths in young children occur in developing countries [Reference Nair2] and are mainly secondary to bacterial infection [Reference Scott3]. However, the increasing availability of molecular diagnostic techniques, confirming the presence of respiratory viruses in at least 50–60% of pneumonia episodes requiring hospitalization [Reference Cevey-Macherel4, Reference Tsolia5], is progressively exposing the significance and burden of these pathogens as a global health threat. Commonly diagnosed respiratory viruses in the developing world include, among others, respiratory syncytial virus (RSV), rhinovirus (RV), human metapneumovirus (hMPV), human bocavirus, influenza and parainfluenza viruses and adenovirus [Reference Ruuskanen6, Reference Lanaspa7]. RSV and hMPV are prominent among viruses due to their high prevalence and association with severe clinical episodes. Indeed, RSV is believed to cause an estimated 3·4 million annual hospital admissions, and between 66 000 and 199 000 deaths, accounting for at least one in every five ARI episodes in children aged <5 years [Reference Nair8]. The burden and consequences of paediatric hMPV infections are much more poorly known, probably on account of its more recent characterization dating back to 2001 [Reference van den Hoogen9], but many reports have clearly documented its high burden, pathogenicity, and comparability to RSV in terms of associated severe outcomes [Reference Ali10–Reference Edwards16].
In this respect, there is a paucity of data available regarding the role of these two viruses in causing ARIs in the Kingdom of Morocco, a middle-income country in North-western Africa. We hereby aim to describe and compare the roles and characteristics of RSV and hMPV in causing WHO-defined severe pneumonia in children aged 2–59 months admitted to a tertiary referral Moroccan paediatric hospital [Reference Jroundi17].
MATERIALS AND METHODS
Study setting and procedures for recruited children
This prospective study was conducted from November 2010 to December 2011 at the Hôpital d'Enfants de Rabat (HER), in Morocco's capital. Children aged 2–59 months admitted to HER with respiratory symptomatology were identified and approached for recruitment if they fulfilled the WHO definition of clinical severe pneumonia (CSP) [Reference Mulholland18, 19], i.e. history of cough or reported breathing difficulty and increased respiratory rate adjusted according to age [19], together with chest indrawing. Children were excluded if the principal reason for hospital admission was a non-respiratory illness or a condition not caused by respiratory illness, or in the event of evidence of a foreign body in the respiratory tract. After parents had signed an informed consent, recruited children underwent standardized procedures upon admission, including pulse oximetry (Bionics Palm Care®, Seoul, South Korea), an antero-posterior chest X-ray, nasal and pharyngeal swabs for diagnosis of bacterial infection/carriage, and a nasopharyngeal aspirate (NPA) for diagnosis of respiratory viruses by molecular techniques. A minimum of 2 ml venous blood was also collected for blood culture, full blood cell count and biochemistry, including C-reactive protein (CRP), and procalcitonin (PCT).
Definitions and clinical groups
All children fulfilled the WHO CSP definition upon admission. However, upon discharge, a study clinician was responsible for reviewing the patient's chart, laboratory and chest X-ray results, and providing a final diagnosis. Discharge diagnoses were coded using ICD-10 [20]. All cases were categorized upon discharge in one of five self-exclusive clinical syndrome groups which included ‘pneumonia’, ‘bronchiolitis’, ‘bronchitis/asthma’, ‘laryngotracheitis (croup)’ and ‘all other diagnoses’. No patient could therefore be classified in more than one group. Hypoxaemia implied an oxygen saturation (SaO2)<90%. Fever was defined as an axillary temperature of ⩾37·5 °C. Nutritional status was based on weight-for-age Z scores (WAZ), calculated using the least mean square method and the 2000 CDC growth chart [21]. Invasive bacterial disease implied the isolation of ⩾1 non-contaminant bacteria in blood or pleural fluid. The Respiratory Index of Severity in Children (RISC) score, a simple and validated clinical score predicting the probability of death in children with lower respiratory tract infections (LRTIs), based on parameters such as oxygen saturation, respiratory signs such as indrawing, or wheezing, and nutritional status [Reference Reed22] was utilized to rank severity in study children. Case-fatality rates (CFRs) were calculated for the different diagnoses as the number of patients who died with that diagnosis divided by the total number of patients with known outcome admitted with that diagnosis. These CFRs represent in-hospital mortality. Cases evaluated at the emergency department but not resulting in hospitalization were excluded.
Laboratory methods
The full laboratory methods of the general study from which this analysis has been performed have been detailed elsewhere [Reference Jroundi17]. Briefly, blood samples were cultured using an automated blood culture system (BD Bactec®, BD, USA), and bacterial isolates identified by Phoenix Automated Microbiology System (PHX system, BD) or colony morphology and biochemical tests. The presence of Streptococcus pneumoniae in NAs and in blood samples was investigated by real-time PCR according to a published home-made assay [Reference Selva23].
Detection of DNA/RNA of hMPV, RSV (A and B), and other viral pathogens [influenza (A and B), parainfluenza virus (1–4), RV, adenovirus, enterovirus and coronavirus (229E, NL63 and OC43)] in NPAs was systematically investigated for all recruited patients by means of the TrueScience® RespiFinder Pathogen Identification Panel (Applied Biosystems, USA), a multiplex PCR-based automated system. This methodology has previously been used in similar populations (children with respiratory symptoms) yielding high positivity rates and virus signatures in around 90% of cases [Reference Mengelle24, Reference Freymuth25]. Careful was taken when processing samples to avoid cross-contamination. Every PCR run included positive and negative controls (as provided in each kit by the manufacturers) to guarantee the reliability of results. In addition, a NPA aliquot from 163 of the patients that tested positive for human RV (the most commonly detected virus in the study population) were shipped to the School of Paediatrics and Child Health (University of Western Australia) in Perth, for quality control, and human RV was successfully genotyped in 157 (96·3%) of those cases. Results of viral molecular testing were not made available to clinicians in charge of the study patients, who decided their clinical management on a case-to-case basis, and according to their clinical judgement.
HIV testing was only performed in patients with clinical signs/symptoms suggestive of immunosuppression, as prevalence of HIV infection in Moroccan children is thought to be <0·5% [Reference Khyatti26].
X-ray interpretation
Chest X-rays were independently interpreted by two paediatricians following a WHO-designed X-ray interpretation protocol [Reference Cherian27]. Discordant results were resolved through a third reading. Evidence of consolidation and/or pleural effusion was defined as ‘endpoint pneumonia’. Other radiological endpoints included interstitial infiltrates or normal radiographs.
Data management and statistical analysis
All study questionnaires were double-entered into a study database using a program written in Filemaker Pro 12 (Filemaker Inc., USA). Statistical analyses were performed with Stata v. 11 (Stata Corp., USA). Study variables were counted and summarized in frequency tables. Qualitative variables were compared using χ 2 test or Fisher's exact test. Means of normally distributed variables were compared using the Student's t test (binary) or ANOVA (more than two categories). For non-normally distributed variables, medians and interquartile ranges are presented, and the Wilcoxon rank sum or other non-parametric tests were used to assess differences. P values <0·05 were considered statistically significant.
Ethics
The protocol and informed consent documents were approved by the Ethics Committee of the Hospital Clinic (Barcelona, Spain), and by the Comité d’Éthique de la Recherche Biomédicale (Départ no. 1252, 16 Déc 2009) of the Faculty of Medicine in Rabat.
RESULTS
Of the 3202 children aged 2–59 months with respiratory symptoms seen at HER during the 14-month study period, only 1334 (42%) were hospitalized, of these 683 (51%) who fulfilled WHO CSP criteria and the rest of the inclusion criteria were included in the final analysis. Nasopharyngeal carriage of at least one respiratory virus in the nasopharynx was almost universal (628/683, 91·9%). Mixed (double, triple, quadruple or even quintuple) infections occurred in 40% of the hospitalized patients. RV was the most commonly detected virus (360 cases, 52·7%), followed by RSV (124 cases, 18·2%) and Adenoavirus (17%), and 61 (8·9%) of inpatients had a hMPV infection. Of RSV-positive infections, 68 (54·8%) were single RSV-A infections, nine (7·3%) single RSV-B infections, and the remaining 47 (37·9%) mixed RSV-A/RSV-B infections. A single case in this series presented with a mixed hMPV-RSV infection, and was excluded from the analysis (Fig. 1). This case occurred in an 11-month-old male child, who was hospitalized for 4 days, did not require intensive care, and received antibiotics on account of a high white blood cell count, high CRP and PCT values, and a chest X-ray compatible with lobar consolidation.
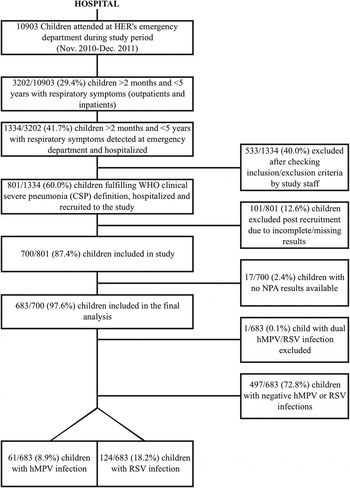
Fig. 1. Study flowchart. HER, Hôpital d'Enfants de Rabat; hMPV, human metapneumovirus; NPA, nasopharyngeal aspirate; RSV, respiratory syncytial virus.
Seasonality
RSV cases presented a very clear seasonal pattern, with over 98% cases occurring between November and April (coinciding with the coldest and least humid months), while hMPV cases were predominantly detected in two peaks, one occurring during the spring months (March–June) and the other from October to December (but only in the second year) (Fig. 2).
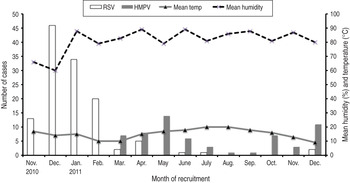
Fig. 2. Number of cases of respiratory syncytial virus (RSV) and human metapneumovirus (hMPV) according to month of recruitment, in relation to humidity and temperature.
Baseline demographics and health history
The baseline socio-demographic and health history factors for each of the two viral groups (RSV vs. hMPV) are shown in Table 1. Of note, hMPV cases were on average older (median age 15 months vs. 10 months), although differences did not reach statistical significance (P = 0·072). Cases of both viruses were otherwise very similar in terms of demographics, patient history, past morbidity and comorbidity, vaccination history, socioeconomic background and family environment.
Table 1. Baseline socio-demographic characteristics and health history of recruited patients with RSV and hMPV infections
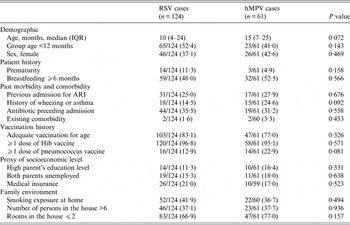
RSV, Respiratory syncytial virus; hMPV, human metapneumovirus; IQR, interquartile range; ARI, acute respiratory infection.
Values given are n (%) unless stated otherwise.
Clinical history, anthropometrics and physical examination findings
Both viral infections showed a few differences in terms of clinical history, anthropometrics, and physical examination findings of the children (Table 2). hMPV cases referred a significantly commoner history of fever and runny nose on admission than RSV cases, and were significantly more frequently pale on arrival (22·9% vs. 9·7%, P = 0·015). In terms of respiratory signs, RSV cases wheezed more (70·2% vs. 52·5%, P = 0·018) and had a significantly higher mean respiratory rate (64·6 vs. 59, P = 0·009).
Table 2. Clinical history, anthropometrics and physical examination findings on admission of recruited patients with RSV and hMPV infections
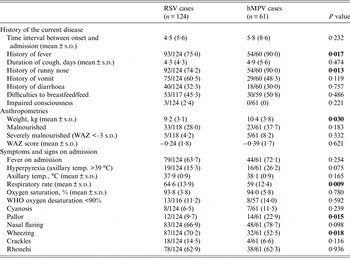
RSV, Respiratory syncytial virus; hMPV, human metapneumovirus; WAZ, weight-for-age Z score.
Values given are n (%) unless stated otherwise.
Laboratory results
Laboratory results also showed some differences between the two groups, with a higher proportion of anaemic patients in the RSV group (19·2% vs. 5·9%, P = 0·032), a significantly lower mean haemoglobin reading on admission (10·4 g/l vs. 12·1 g/l, P = 0·004) while hMPV cases presented a higher proportion of elevated CRP (>5 mg/dl) (36·1% vs. 21·8%, P = 0·041). hMPV cases were twice as frequently infected with pneumococci in their nasopharynx (29·5% vs. 12·9%, P = 0·006), and were significantly more often viral mono-infections than RSV cases (49·2% vs. 29·0%, P = 0·007). Both viruses co-existed frequently with other respiratory viruses (RV, coronavirus, influenza, parainfluenza virus or adenovirus), with only parainfluenza co-infections being more commonly seen in hMPV cases (14·7% vs. 5·6%, P = 0·038) (Table 3).
Table 3. Laboratory and microbiology findings of recruited patients with RSV and hMPV infections
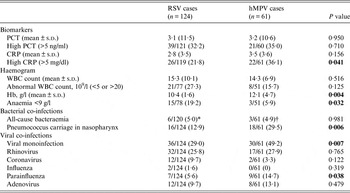
RSV, Respiratory syncytial virus; hMPV, human metapneumovirus; PCT, procalcitonin; CRP, C-reactive protein; WBC, White blood cell.
Values given are n (%) unless stated otherwise.
* Staphylococcus aureus (n = 2), Pseudomonas aeruginosa (n = 1), Pseudomonas oryzihabitans (n = 1), Escherichia coli (n = 1), Streptococcus pneumonia (n = 1).
† Enterococcus faecalis (n = 1), Streptococcus pneumoniae (n = 1), Streptococcus group C (n = 1).
Clinical evolution and outcome, chest X-ray findings and management
Regarding clinical syndromes, RSV was more commonly associated to bronchiolitis episodes (41·1% RSV vs. 6·6% hMPV, P < 0·001), while the reverse was seen in the associations with laryngotracheitis (hMPV 9·8%, RSV 0%, P < 0·001). Importantly, although hMPV was more frequently associated with pneumonia episodes (42·6% vs. 31·4%, P = 0·135) or bronchitis/asthma (39·3% vs. 25·8%, P = 0·060) none of these differences showed statistical significance. Chest X-ray findings were also similar between the two groups (Table 4). In terms of management, RSV-infected patients received more bronchodilator treatment (P = 0·011), while hMPV patients received more corticosteroids (P = 0·001) and antibiotics (P = 0·048). There were no differences in oxygen needs or in mean duration of admission, but hMPV patients had a higher mean RISC score (1·8 vs. 1·5, P = 0·025), and were more commonly transferred to the intensive care unit (ICU) (11·5% vs. 3·2%, P = 0·026). There were seven deaths in the study, evenly distributed between the two groups (4/124 in the RSV group, with two cases of pneumonia, one of bronchiolitis and one of bronchitis; and 3/59 in the hMPV group, with two cases of pneumonia, one bronchiolitis; P = 0·540). No statistically significant differences could be detected between the deaths in each group.
Table 4. Syndromic diagnosis, radiology endpoints and outcome of recruited patients with RSV and hMPV infections
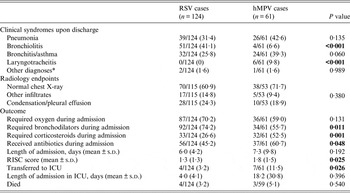
RSV, Respiratory syncytial virus; hMPV, human metapneumovirus; RISC, Respiratory Index of Severity in children; ICU, intensive care unit.
Values given are n (%) unless stated otherwise.
* Miscellaneous group of diseases including upper respiratory tract infections, other respiratory conditions or infections, congenital problems, other infections.
DISCUSSION
This analysis, part of a larger study describing for the first time the aetiology and epidemiology of WHO-defined pneumonia in Morocco [Reference Jroundi17], confirms the high prevalence and importance in this country of both RSV and hMPV infections as causes of paediatric respiratory-related hospital admissions. Such data are of importance at the national level, but even more so at the regional level in Northern Africa, where few studies have specifically investigated and compared these two common viruses [Reference Fodha, Legrand and Vabret28–Reference Qaisy32]. In our series, RSV infections were documented in 18·2% of all recruited children, whereas hMPV infections were half as common (8·9% of all patients). Importantly, and despite the high frequency of co-infections between RSV and hMPV with other common respiratory viruses, only one case of RSV/hMPV co-infection could be confirmed. This, together with the fact that a third of all RSV cases and up to a half of all hMPV infections occurred in the absence of other viral co-infections, confirms the pathogenicity of these two viruses and their potential to cause severe disease, with non-negligible associated CFRs ranging from 3·2% (RSV) to 5·1% (hMPV). However, the real relevance of the isolation of a pathogen in the nasopharynx (viral or bacterial) in causing the clinical picture in those patients is still debatable, as asymptomatic carriage is also a common phenomenon.
It has been clearly demonstrated that although young age is an independent risk factor for both pathogens [Reference Papenburg and Boivin33, Reference Mullins34], hMPV occurs both in infancy and also at higher frequency than in RSV cases, in older children and at a higher mean age [Reference Eggleston11, Reference Paget12, Reference Edwards16]. In our series, mean age on admission was also higher for hMPV patients, although statistical significance could not be achieved (P = 0·07). We were unable to find any other distinguishing features between the two viruses in terms of demographics, patient's history, vaccination history, family environment or socioeconomic background, and contrary to what other authors have described, we did not find differences in terms of underlying chronic comorbidities [Reference Eggleston11, Reference Papenburg35]. What seemed to differ quite noticeably, as shown previously [Reference Paget12, Reference Wilkesmann14, Reference Edwards16, Reference McCracken36], was the seasonal pattern of both viruses, with RSV showing a clear-cut transmission period coinciding with the cold and drier season (November–April) with hMPV concentrated in the remaining months of the year (March–December), with very little overlap.
It has also been argued that the clinical manifestations of hMPV are indistinguishable from those of RSV [Reference Papenburg and Boivin33]. However, our analysis does pinpoint a few differences regarding the medical history and physical examination on admission, such as a higher frequency of children with runny nose or a history of fever for hMPV cases. Conversely, RSV-infected patients had significantly higher respiratory rates or greater frequency of wheezing than their comparator hMPV cases. Such minor differences may be linked to the fact that, in our series, and as described previously by other studies, RSV was significantly more frequently associated with bronchiolitis (41·1% vs. 6·6%, P < 0·001) [Reference Papenburg35, Reference Boivin37], while hMPV was more related to laryngotracheitis (P < 0·001) and (albeit non-significantly) to pneumonia [Reference Wilkesmann14, Reference Papenburg35]. Similarly, although our data cannot support the hypothesis that hMPV may predispose or simply be more frequently associated with the risk of invasive bacterial disease, two findings should be mentioned in this respect, i.e. the significantly higher frequency of elevated (>5 mg/dl) CRP (P = 0·041) and pneumococcal nasopharyngeal carriage (P = 0·006) in hMPV-infected patients. Conversely, the low detection of pneumococci in the nasopharynx of RSV-infected patients (only 12·9% of cases) is of note, as some authors have proposed that RSV could be a predisposing agent for secondary bacterial infection in the airways of children [Reference Korppi38–Reference Zhou40]. However, and similarly to what other authors have found [Reference Launes41], our data do not confirm a major synergism between pneumococcus and RSV, and further studies are needed to clarify the real interaction between these two pathogens, particularly using better sampling methods that may be more helpful to disentangle the real contribution of isolates, such as lung aspirates [Reference Carrol42, Reference Howie43], or minimally invasive autopsies of the lung in post-mortem samples [Reference Hart44].
Despite mirroring quite closely the clinical presentation of RSV episodes on admission, hMPV infections are associated with a higher risk than RSV infections in terms of disease progression and hospital requirements. In our series, hMPV-infected patients were significantly more prone to receive antibiotics (P = 0·048), corticosteroids (P = 0·001, possibly in relation to their more frequent association with laryngotracheitis instead of with bronchiolitis), and transfer to the ICU (P = 0·026), and had a significantly higher mean RISC score (1·8 vs. 1·3, P = 0·025), suggesting a higher degree of severity and intra-hospital complications. Importantly, clinicians were not aware at the time of deciding upon the clinical management of these patients which viruses (if any) had been detected in the NPA of these children, and were causing the respiratory syndrome. This aligns with a previous analysis of this same series of patients in which hMPV infection was found to be the only independent microbiological risk factor associated with an adverse outcome [Reference Jroundi45].
CONCLUSIONS
We have shown for the first time that both RSV and hMPV are common and potentially life-threatening causes of WHO-defined pneumonia in Moroccan children. Both viruses show indistinctive clinical symptomatology, but in our series, hMPV had a number of characteristics associated with a more severe evolution. Further studies are warranted to better characterize the nature and risk factors for these infections, in order to improve their early recognition and to guarantee better preventive and management strategies.
ACKNOWLEDGEMENTS
The authors acknowledge the facilitating role played by Pascal Andignac, Eva López, Younes Ben Azzouz, Maria José López, Robert Álvarez and the rest of the team at Fundació Clínic Maroc, together with the nursing and clinical personnel at HER, Rabat (Emergency ward, P1 and ICU), and the technical personnel at the research laboratory of CHIS. We also thank Dr Jordi Vila and Dr Míriam Álvarez, who contributed to the general set up of laboratory methods at the research laboratory. Edward B. Hayes played a critical role in the design of the study protocol. Finally, we are grateful to the mothers, guardians and study participants.
This study received funding from the Spanish Agency of International Cooperation for Development (AECID) through grant 07-CO1-021 awarded to Fundació Clínic per a la Recerca Biomèdica (Convenio de Fortalecimiento del sistema nacional de salud, con especial atención a la salud materno-infantil, Marruecos, 2008–2012). The research leading to these results also received funding from the People Programme (Marie Curie Actions) of the European Union's Seventh Framework Programme FP7/2007–2013 under REA grant agreement no. 621216. J.R. has a fellowship from Program I3, of the ISCIII (grant no.: CES11/012). Q.B. has a fellowship from the program Miguel Servet of the ISCIII (Plan Nacional de I + D + I 2008–2011, grant no.: CP11/00269).
DECLARATION OF INTEREST
None.











