Zn and Mg are essential minerals involved in functioning of the central nervous system. Dietary sources of Zn include red meat, poultry, legumes, nuts and seeds, certain types of seafood (e.g. oysters, crab and lobster), whole grains, fortified breakfast cereals and dairy products. Mg is widely distributed in plant foods, particularly green leafy vegetables, legumes, nuts, seeds and whole grains. Zn is a cofactor of many enzymes that play a role in brain function( Reference Frederickson, Koh and Bush 1 ) and is present in regions of the brain associated with the pathophysiology of mood disorders, including the amygdala, hippocampus and cerebral cortex( Reference Takeda 2 ). Zn modulates neuronal excitability by inhibiting both the GABA (γ-aminobutyric acid) and NMDA (N-methyl-d-aspartate) receptors( Reference Smart, Xie and Krishek 3 ) and has shown antidepressant-like activities in animal models( Reference Franco, Posser and Brocardo 4 – Reference Szewczyk, Poleszak and Sowa-Kucma 6 ). Mg is another potent antagonist of the NMDA receptor complex( Reference Morris 7 ) and Mg deficiency has been related to symptoms such as agitation, anxiety, irritability and hyperexcitability( Reference Eby and Eby 8 ). In rodent models, Mg depletion increases anxiety and depression-like behaviours( Reference Singewald, Sinner and Hetzenauer 9 , Reference Whittle, Li and Chen 10 ), and mice with low erythrocyte Mg levels have been found to exhibit more aggressive behaviour than those with high Mg levels( Reference Henrotte, Franck and Santarromana 11 ).
Recently, Jacka et al. reported that dietary intakes of Zn and Mg were inversely and cross-sectionally associated with depressive and anxiety scores in a population-based sample of women (n 1046)( Reference Jacka, Maes and Pasco 12 ). Other studies have shown inverse relationships between dietary Zn intakes and depression in women( Reference Amani, Saeidi and Nazari 13 – Reference Sawada and Yokoi 16 ). Furthermore, research suggests that Zn supplementation as an adjunct to antidepressant drug treatment significantly lowers depressive symptoms in depressed patients compared with antidepressant treatment alone( Reference Nowak, Siwek and Dudek 17 – Reference Ranjbar, Kasaei and Mohammad-Shirazi 19 ). Research on Mg with depressive and anxiety symptoms is less conclusive. Although energy-adjusted Mg intakes were inversely associated with depression scores in a sample of Norwegian community-dwelling men and women (n 5708)( Reference Jacka, Overland and Stewart 20 ), this finding was not supported in a cohort (n 12939) of Spanish university graduates( Reference Derom, Martinez-Gonzalez and Sayon-Orea Mdel 21 ). Zn and Mg supplementation have both been shown to be beneficial in the treatment of attention-deficit hyperactivity disorder (ADHD) in children, as a stand-alone treatment and as an adjunct to medication( Reference Akhondzadeh, Mohammadi and Khademi 22 – Reference Mousain-Bosc, Roche and Polge 24 ); however, limited research exists in this area.
While there is increasing recognition of the role of Mg and Zn in mental health, most of the evidence to date has focused on adult participants, often with a cross-sectional design or in the context of a clinical trial. In the present study we aimed to examine the prospective association between dietary intakes of Zn and Mg and internalising (withdrawn, somatic complaints, anxious/depressed) and externalising (attention problems, aggressive/delinquent) behaviour problems in a population-based cohort of adolescents at the 14- and 17-year follow-ups. Our hypothesis was that lower dietary intakes of Zn and Mg would be associated with increased internalising and externalising behaviour problems.
Methods
Participants
The Western Australian Pregnancy Cohort (Raine) Study methodology has been described previously( Reference Newnham, Evans and Michael 25 ). In brief, a total of 2900 pregnant women attending the public antenatal clinic at King Edward Memorial Hospital, or nearby private practices, were recruited into the Raine Study between May 1989 and November 1991 and gave birth to 2868 children. These children underwent assessment at birth and at regular intervals. Recruitment and all follow-ups were approved by the ethics committees of King Edward Memorial Hospital for Women and the Princess Margaret Hospital for Children, Perth, Western Australia. Informed and written consent was obtained from the participant and/or their primary caregiver for all follow-ups. Data collection for the 14- and 17-year follow-ups occurred between 2003–2005 and 2006–2008, respectively.
Assessment of mental health
Mental health at 14 and 17 years was assessed using the Youth Self-Report (YSR), which is a version of the Child Behaviour Checklist for Ages 4–18 (CBCL/4-18) and is designed specifically for self-report in adolescents. The YSR is a 118-item, empirically validated and reliable measure of emotional and behavioural problems in children and adolescents( Reference Achenbach 26 , Reference Achenbach and Rescorla 27 ). The YSR generates an externalising problem score that describes uncontrolled and antisocial behaviour (attention problems, aggressive/delinquent) and an internalising problem score that describes over-controlled and inhibited behaviour (withdrawn, somatic complaints, anxious/depressed), with higher scores indicating a higher level of emotional and behavioural problems. We calculated standardised T-scores for total, externalising and internalising problem scales, normalised separately for boys and girls by age.
Assessment of zinc and magnesium intakes
A semi-quantitative FFQ developed by the Commonwealth Scientific and Industrial Research Organisation (CSIRO) in Adelaide, Australia was used to assess Zn and Mg intakes( Reference Baghurst and Record 28 ). This 212-item FFQ assessed usual dietary intake over the previous year, collecting information on the frequency of consumption of individual foods, mixed dishes and beverages, along with information on usual serving sizes in relation to a standard serving size in household units. Seasonal differences were accounted for by asking how often foods were eaten in summer and winter. At the 14-year follow-up, the primary caregiver was asked to complete the FFQ in association with the adolescent. At the 17-year follow-up, the FFQ was completed by the adolescent. The questionnaire has been validated against a 3 d food record in the same cohort at the 14-year follow-up( Reference Ambrosini, de Klerk and O’Sullivan 29 ).
All questionnaires were checked by a research nurse and queries were clarified with the adolescent or primary caregiver. Food intake data were entered into a database and verified by CSIRO. Estimated daily micronutrient intakes were provided by CSIRO using nutrient composition derived from four sources: (i) the Australian nutrient database (NUTTAB95)( Reference Lewis and Hunt 30 ); (ii) the British food composition tables( Reference Holland, Unwin and Buss 31 ); (iii) the US Department of Agriculture food tables( 32 ); and (iv) manufacturers’ data. Questionnaires were excluded if the daily energy intake reported was implausible (<3000 or >20 000 kJ/d).
Potential confounding variables
Participants were weighed to the nearest 100 g using a Wedderburn digital chair scale and height was determined to the nearest 0·1 cm with a Holtain stadiometer. BMI was calculated as weight in kilograms divided by the square of height in metres. Dietary misreporting was estimated using the Goldberg method( Reference Poslusna, Ruprich and de Vries 33 ), which is widely used to estimate the cut-off levels for under-reporters, plausible reporters and over-reporters in dietary surveys. Current use of nutritional supplements (yes/no) was collected from a self-reported questionnaire.
Physical activity was assessed using a self-reported questionnaire based on exercise outside of school hours per week, with exercise defined in three categories for activity causing breathlessness or sweating (≥4 times/week, 1–3 times/week and <1 time/week). Annual family income before tax was completed by the primary caregiver and reported in three categories (≤$AUS 40000, $AUS 40001–78000 and >$AUS 78000). Family functioning was included in order to account for a number of related family factors, including communication and parental conflict, allowing for a parsimonious model while also considering the importance of family in offspring mental health. Family functioning was measured using the twelve-item General Functioning Scale (GFS) from the McMaster Family Assessment Device( Reference Epstein, Baldwin and Bishop 34 ). The scale has been shown to be reliable and internally consistent( Reference Byles, Byrne and Boyle 35 ), with lower scores on the GFS representing poorer family functioning and higher scores representing better family functioning.
Statistical analysis
Characteristics of participants who completed the FFQ and the YSR at both 14 and 17 years were compared with non-participants from the original cohort. Sex, race, family income during pregnancy, maternal age at birth, maternal education and maternal pre-pregnancy BMI were compared using χ 2 tests. Baseline characteristics were described for participants in the current study, including sex, YSR T-scores (total, internalising and externalising), Zn and Mg intakes, energy intake, dietary misreporting, supplement use, BMI, physical activity, family income and family functioning.
Zn and Mg intakes at 14 and 17 years were converted to Z-scores within each follow-up separately. General linear mixed models were used to investigate the prospective univariate relationships between Zn and Mg intakes and YSR T-scores (total, internalising and externalising). Models were then adjusted for sex, physical activity, family income, supplement status, dietary misreporting, BMI, family functioning and energy intake. Interactions between time and Zn or Mg intakes were explored in order to determine whether the effect of intakes on the YSR T-scores were different at the two follow-ups. Similarly, interactions between sex and Zn or Mg intakes were investigated in order to determine whether there were sex differences in the effect of Zn and Mg intakes on YSR T-scores. Analyses were performed using the statistical software package IBM SPSS Statistics Release Version 19·9·9·1. Statistical significance was defined as P<0·05.
Results
A total of 684 adolescents completed the YSR and FFQ at both follow-ups (Fig. 1). Compared with those from the original cohort who did not participate in the current study (n 2184), participants were more likely to be female, Caucasian, to come from families with a higher income during pregnancy and to have mothers with a higher age, higher education during pregnancy and healthier pre-pregnancy BMI (P<0·05).
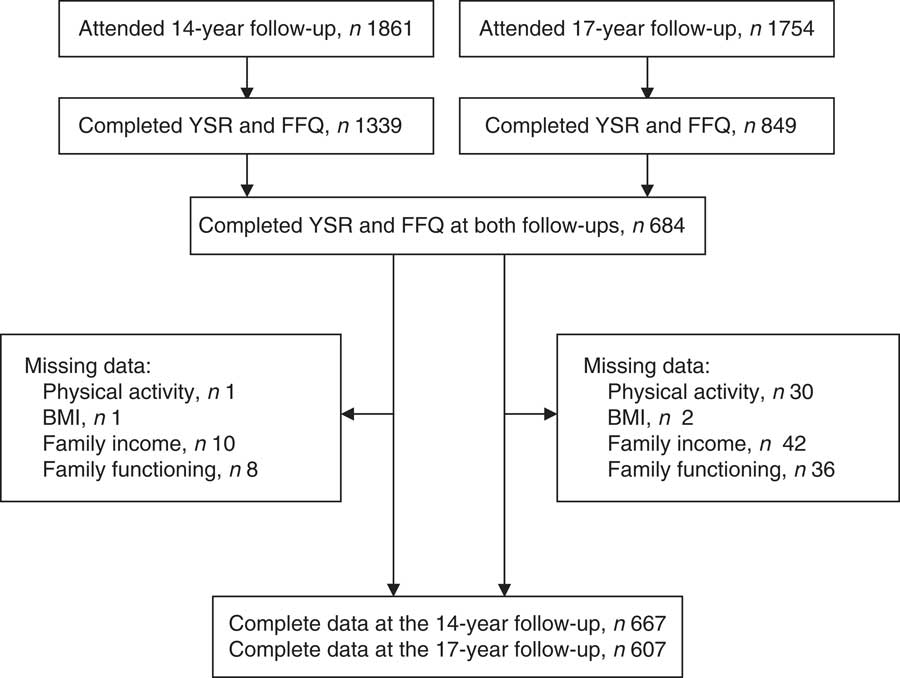
Fig. 1 Flow diagram of adolescents attending the 14- and 17-year follow-ups, Western Australian Pregnancy Cohort (Raine) Study (YSR, Youth Self-Report)
Mean YSR total, internalising and externalising T-scores were approximately 50 at both follow-ups (Table 1), which is consistent with population norms. Dietary Zn and Mg intakes were similar at 14 and 17 years: mean Zn intake was approximately 12 mg/d and Mg intake was approximately 310 mg/d. The mean intakes in this population were in line with the Estimated Average Requirements for Zn and Mg (Zn, 11 mg/d for males and 6 mg/d for females; Mg, 340 mg/d for males and 300 mg/d for females).
Table 1 Characteristics of the Western Australian Pregnancy Cohort (Raine) Study participants for whom YSR and micronutrient intakes were available at both the 14- and 17-year follow-ups (n 684)
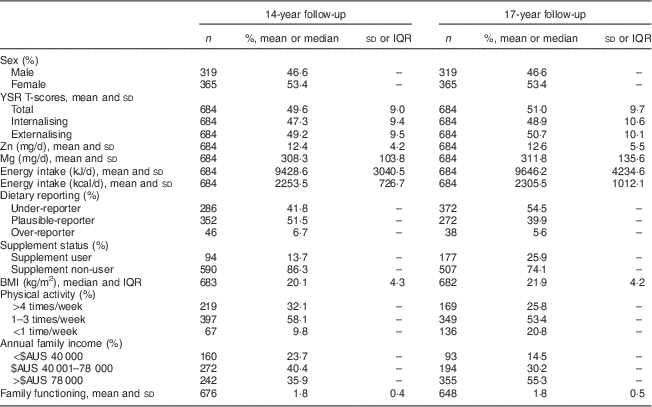
YSR, Youth Self-Report; IQR, interquartile range.
In univariate analyses (n 684), Zn and Mg intakes (per sd increase) were not significantly associated with internalising or externalising behaviours over the 3-year study period (Table 2). However, after adjusting for potential confounders (n 667 at 14 years and n 607 at 17 years), including sex, physical activity, family income, supplement use, dietary misreporting, BMI, family functioning and energy intake, there was a significant inverse association between Mg and externalising behaviour problems. Although there was a trend towards improved externalising behaviour problems with increased Zn intake, the association did not reach the statistically significant level we had specified. There were no significant interactions between time and Zn or Mg intakes, or between sex and Zn or Mg intakes. Further, there were no significant associations between Zn and Mg intakes and internalising behaviour problems or total YSR T-scores.
Table 2 General linear mixed model coefficients for YSR T-scores and zinc and magnesium intakes at ages 14 and 17 years, Western Australian Pregnancy Cohort (Raine) Study
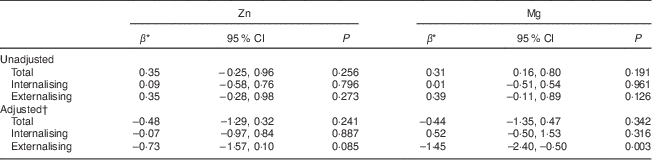
YSR, Youth Self-Report.
* Estimated difference in YSR T-scores per sd increase in Zn and Mg intakes.
† Adjusted for sex, physical activity, family income, supplement status, dietary misreporting, BMI, family functioning and energy intake; n 667 at 14 years, n 607 at 17 years.
Discussion
The current study examined the prospective associations between Zn and Mg intakes and internalising and externalising behaviour problems in adolescents. The results support our hypothesis that dietary Mg intakes are inversely associated with externalising behaviour problems in adolescents. Although we found no significant associations between Zn and internalising or externalising behaviours, there was a trend towards reduced externalising behaviour problems with higher Zn intakes. Externalising behaviour problems include attention problems, rule-breaking behaviours and aggressive behaviours, meaning that there is some overlap between our findings and previous research that found beneficial effects of Mg supplementation on symptoms of ADHD( Reference Akhondzadeh, Mohammadi and Khademi 22 – Reference Mousain-Bosc, Roche and Polge 24 ). However, we did not find a significant association between Zn or Mg intake and internalising behaviour problems, which contrasts with previous literature( Reference Jacka, Maes and Pasco 12 – Reference Maserejian, Hall and McKinlay 15 , Reference Nowak, Siwek and Dudek 17 , Reference Siwek, Dudek and Paul 18 , Reference Jacka, Overland and Stewart 20 ).
There are several reasons why Mg intake may relate to externalising behaviour problems in adolescents. Some insight is provided by considering symptoms of ADHD, which share characteristics of externalising behaviours and may also be influenced by intake of Mg. Mg plays a role in the function of the serotonergic, noradrenergic and dopaminergic receptors, which are related to the pathophysiology of ADHD( Reference Cardoso, Lobato and Binfare 36 ). Improved ADHD symptoms have been reported in children after Mg supplementation( Reference Mousain-Bosc, Roche and Polge 24 , Reference Starobrat-Hermelin and Kozielec 37 ).
We found no prospective association between dietary Zn and internalising behaviour problems (withdrawn, somatic complaints, anxious/depressed) in our study, which is consistent with results from a longitudinal study of 2317 middle-aged Finnish men( Reference Lehto, Ruusunen and Tolmunen 38 ). In contrast, dietary intakes of Zn have repeatedly shown an inverse relationship with depressive symptoms in cross-sectional analyses, particularly in women( Reference Jacka, Maes and Pasco 12 – Reference Maserejian, Hall and McKinlay 15 , Reference Davison and Kaplan 39 – Reference Yary and Aazami 41 ). We also found no association between dietary Mg intakes and internalising behaviour problems, which is consistent with a study in 12 939 Spanish university graduates( Reference Derom, Martinez-Gonzalez and Sayon-Orea Mdel 21 ). However, a number of cross-sectional studies have found an inverse association between dietary Mg intakes and depressive symptoms( Reference Jacka, Maes and Pasco 12 , Reference Jacka, Overland and Stewart 20 , Reference Davison and Kaplan 39 , Reference Jung, Ock and Chung 42 , Reference Yary, Aazami and Soleimannejad 43 ). Differences between our results and those reported elsewhere may stem, at least in part, from differences in the age of the participants and the nature and duration of follow-up. Our study included population-based adolescents followed from 14 to 17 years, whereas most of the previous literature has included adult participants, often assessed cross-sectionally (in the case of population-based studies) or in the context of a clinical trial. Further research is warranted to determine if Zn and Mg intakes relate to internalising problems in some age or demographic groups and not others.
The equivocal results in studies examining dietary Zn and Mg intakes and mental health may also relate to the use of different mental health assessment tools. An advantage of our study is the use of the YSR, since it distinguishes between internalising and externalising behaviour problems; a differentiation that is not captured by all mental health measures. At the same time, the YSR does not generate clinical diagnoses and we cannot comment on associations between dietary intakes and clinically significant depression, anxiety or ADHD. Differences in how mental health problems are conceptualised and assessed may contribute to differences in results across studies, again speaking to the need for further research in this area.
Strengths of our study were the prospective study design, use of a validated FFQ and extensive characterisation of a population-based cohort. The latter allowed us to assess the effect of dietary Zn and Mg intakes on mental health while accounting for a wide range of potential confounding factors. A limitation of our study was the use of a self-reported questionnaire, rather than clinical diagnosis, to assess mental health problems. While self-report assessment of mental health may lead to more truthful reporting than face-to-face assessment, self-report measurements are subject to reporting bias. It can also be difficult to accurately assess nutrient intakes using an FFQ. However, the FFQ used in the present study was validated against a 3 d food record in the same cohort and the mean daily intakes of Zn and Mg were similar when measured by the FFQ and the 3 d food record( Reference Ambrosini, de Klerk and O’Sullivan 29 ).
It is possible that behaviour problems result in altered appetite and eating habits, including increased consumption of processed foods, which are lower in minerals such as Zn and Mg. Furthermore, growing evidence suggests that obesity may be related to numerous psychiatric disorders and several behavioural and biological pathways have been proposed to explain this potential relationship, which are outside the realm of nutrition( Reference Marsha and Wildes 44 ). In adjusting for confounders, we have attempted to present evidence for a causative relationship; however, we cannot rule out the possibility of reverse causality or residual confounding. A further limitation of the study was the loss to follow-up. Participants included in the current study were more likely to be from families with higher socio-economic status relative to participants from the original cohort and care should be taken when generalising results to the wider community. However, although attrition may have been higher for those participants suffering mental health difficulties, the YSR T-scores in the current study reflect the population norm.
Given that dietary Mg intake can be optimised through the consumption of nutrient-dense foods and supplementation, our study has important public health implications. Promoting increased consumption of Mg-rich foods, such as green leafy vegetables, legumes, nuts, seeds and whole grains, along with supplementation to address identified micronutrient deficiencies, may be a useful strategy to prevent mental health and behavioural problems in adolescents. In order to determine any benefit of Mg and/or Zn supplementation in the prevention and treatment of externalising behaviour problems, further randomised controlled trials using optimal doses are necessary.
Acknowledgements
Acknowledgements: The authors gratefully acknowledge the Raine Study participants and their families, and the Raine Study Team, for cohort coordination and data collection. Financial support: Core funding for the Western Australian Pregnancy Cohort (Raine) Study is provided by the University of Western Australia; the Faculty of Medicine, Dentistry and Health Sciences at the University of Western Australia; the Telethon Kids Institute; the Women and Infants Research Foundation; Curtin University; and the Raine Medical Research Foundation. Specific data collection for the 14-year follow-up was funded by the National Health and Medical Research Council (project grant ID 211912). Data collection at the 17-year follow-up was funded by the National Health and Medical Research Council (programme grant ID 353514 and project grant ID 403981). The authors thank the Telstra Research Foundation, the West Australian Health Promotion Foundation, the Australian Rotary Health Research Fund, the National Heart Foundation of Australia/Beyond Blue and the National Health and Medical Research Council (project grant ID 634445; project grant ID 1022134; programme grant ID 003209) for their provision of further funding for investigator and data support. The funders had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: L.J.B. conducted the data analysis, interpreted the data, contributed to the intellectual content of the manuscript and wrote the manuscript; P.J. coordinated the statistical analysis, interpreted the data, contributed to the intellectual content of the manuscript and contributed to the manuscript preparation; K.L.A. contributed to the intellectual content of the manuscript and reviewed the final manuscript; G.S.T. contributed to the intellectual content of the manuscript and reviewed the final manuscript; C.M.G. contributed to the intellectual content of the manuscript and reviewed the final manuscript; S.M.B. contributed to the intellectual content of the manuscript and reviewed the final manuscript; W.H.O. contributed to the intellectual content of the manuscript, the manuscript preparation and obtained funding for the study. Ethics of human subject participation: Recruitment and all follow-ups were approved by the ethics committees of King Edward Memorial Hospital for Women and the Princess Margaret Hospital for Children, Perth, Western Australia.







