Se is an essential micronutrient for animals and humans required for normal growth and development( Reference Rayman 1 , Reference Papp, Lu and Holmgren 2 ). This role is attributed to low molecular weight Se compounds, as well as to the presence of Se within proteins and enzymes, named selenoproteins, in the form of the amino acid selenocysteine. Mammals have at least twenty-five selenoproteins, and fish probably more than thirty-two, although the functions of many selenoproteins are not yet elucidated( Reference Brigelius-Flohé 3 , Reference Lobanov, Hatfield and Gladyshev 4 ). Selenoproteins are involved in diverse physiological functions such as antioxidant defence, reduction of inflammation, thyroid hormone production, DNA synthesis, fertility and reproduction( Reference Rayman 1 ). As a component of glutathione peroxidases (GPX), thioredoxin reductases (TR), and methionine sulphoxide reductases (MSR), Se plays, in particular, a pivotal role against oxidative cellular injury and lipid peroxidation. GPX can reduce H2O2 and organic hydroperoxides to the corresponding alcohols with oxidation of glutathione to glutathione disulphide( Reference Arthur 5 ). Seven GPX have been described in mammals, five of which are selenoproteins( Reference Papp, Lu and Holmgren 2 ). The GPX selenoproteins include the ubiquitously expressed cytosolic GPX1, a gastrointestinal-specific enzyme GPX2, a secreted protein found in plasma GPX3, a ubiquitously expressed enzyme that acts on oxidised lipids named phospholipid hydroperoxide GPX or GPX4, and the GPX6 located in olfactory epithelium and embryonic tissues. GPX1 and GPX4 genes have been cloned in rainbow trout revealing several isoforms( Reference Pacitti, Wang and Page 6 ). TR can act by controlling the function of thioredoxin, a central redox molecule, and by directly reducing numerous substrates including lipid hydroperoxides( Reference Papp, Lu and Holmgren 2 ). Three Se-containing TR have been identified in mammals with TR1, the most abundant isoenzyme found in the cytosol, TR2, a mitochondrial form and TR3 found predominantly in the testis( Reference Crosley, Méplan and Nicol 7 ). MSR repair oxidatively damaged proteins and protect against oxidative stress( Reference Kim 8 ). MSR include two stereospecific enzyme families: MSRA specific for reduction of the S-epimer of methionine sulphoxide, and MSRB which can only act on the R-form. Three selenoprotein MSRB genes have been identified in mammals with MSRB1 also known as selenoprotein R or X present in the cytosol and nucleus, MSRB2 targeted to the mitochondria and MSRB3 localised in the endoplasmic reticulum. In addition, selenoprotein P (SelP), present in the plasma of mammals and expressed in cellular membranes, is regarded as a transport protein for Se but also has antioxidant properties, as it can directly reduce phospholipid hydroperoxides in vitro ( Reference Takebe, Yarimizu and Saito 9 , Reference Steinbrenner, Bilgic and Alili 10 ).
Dietary Se deficiency can influence the level of activity of several selenoproteins, and in some cases also cause changes in mRNA levels for selenoprotein genes( Reference Brigelius-Flohé 3 , Reference Hesketh 11 , Reference Mariotti, Ridge and Zhang 12 ). If Se intake is suboptimal, selenoprotein functions may be impaired, resulting in altered Se metabolism. In fish, a dietary Se deficiency has generally been reported to result in reduced activity of GPX, as well as growth reduction( 13 ) and increased mortality( Reference Wang, Peng and Huang 14 ). Muscular dystrophy and exudative diathesis have also been observed in channel catfish, when dietary Se deficiency was combined with dietary vitamin E deficiency( Reference Gatlin, Poe and Wilson 15 ). On the other hand, Se may exert toxic effects at levels marginally above those required( 13 , Reference Watanabe, Kiron and Satoh 16 ). A dietary Se requirement varying from 0·15 to 0·7 mg/kg diet has been reported for different fish species based on weight gain and GPX activity with threshold levels for adverse effects estimated at 3–4 mg Se/kg diet( 13 ). This dietary Se requirement might be higher in early life stages of fish exhibiting a high growth rate as suggested by Bell et al. ( Reference Bell, Cowey and Adron 17 ) to explain the absence of pathology in rainbow trout juveniles, compared to Atlantic salmon fry fed low dietary Se levels. The chemical form of Se is known to strongly affect its bioavailability and its impact on metabolism( Reference Pacitti, Wang and Page 6 , Reference Jaramillo, Peng and Gatlin DM 18 , Reference Rider, Davies and Jha 19 ). Animal feeds can be supplemented with inorganic (e.g. selenite, selenate) or organic (e.g. selenomethionine, selenocysteine) Se to a maximum authorised level of 0·3 mg/kg according to US Food and Drug Administration( 20 ), and 0·2 mg/kg according to European Food Safety Authority( 21 ). Fish meal-based diets generally provide sufficient Se to satisfy the nutritional requirements of fish( Reference Watanabe, Kiron and Satoh 16 ), but can contain levels of Se well above the maximum dietary levels permitted by European Food Safety Authority (0·5 mg/kg). However, currently, there is significant reduction in fishmeal levels in fish feeds, particularly in salmonid feeds, replaced to a large extent by plant ingredients( Reference Tacon and Metian 22 ) that vary widely in their Se content( Reference Watanabe, Kiron and Satoh 16 , Reference Rayman, Infante and Sargent 23 ).
The objective of the study was hence to assess the impact of dietary Se supplementation by an organic or inorganic source on the antioxidant status of rainbow trout (Oncorhynchus mykiss) fry fed practical plant protein or fishmeal-based feeds.
Materials and methods
Diets
Ingredients were mixed and the diets were manufactured using a twin-screw extruder (BC 45, Clextral) at the INRA experimental facilities in Donzacq (Landes, France). Six diets were formulated with two different basal ingredient compositions (Table 1). P-diets were based on plant-derived proteins and vegetable oils, and F-diets were formulated to contain fishmeal and fish oil as protein and lipid sources. The P-diets were supplemented with 1 % mineral mixture to meet all the essential mineral requirements of rainbow trout( 13 ), except for Se. F-diets were not supplemented with mineral mixture, as fishmeal represents a major source of minerals and trace elements; high Ca or P levels have been shown to adversely affect absorption of some trace elements in different fish species( 13 ); high levels of other trace elements such as Cu and Zn have been shown as well to interfere with Se( Reference Naganuma, Tanaka and Maeda 24 – Reference Feroci, Badiello and Fini 26 ). SeS- and SeY-diets were supplemented with either sodium selenite (SeS-diets) or the Se-enriched yeast Selsaf (SeY-diets) at a concentration of 0·3 mg Se/kg feed. PSe0- and FSe0-diets containing 0·5 and 1·2 mg Se/kg feed respectively, were not supplemented with Se and served as controls.
Table 1 Formulation and composition of the experimental diets (g/100 g dry weight)

PSe0, plant-based diet not supplemented with Se; PSeS, plant-based diet supplemented with sodium selenite; PSeY, plant-based diet supplemented with Se-enriched yeast; FSe0, fishmeal-based diet not supplemented with Se; FSeS, fishmeal-based diet supplemented with sodium selenite; FSeY, fishmeal-based diet supplemented with Se-enriched yeast.
* Plant meals (% diet): 20 % wheat gluten (Roquette); 18 % corn gluten meal (Inzo); 15 % soyabean protein concentrate Estrilvo 70 (Sopropêche); 6·2 % soyabean meal (Sud-Ouest Aliment); 5·2 % rapeseed meal 00 (Sud-Ouest Aliment); 5 % white lupin meal (Terrena); 3·8 % dehulled pea meal Primatex (Sotexpro); 1·3 % l-lysine (Ajinomoto-Eurolysine); 0·3 % l-methionine (Evonik).
† Norwegian herring meal Norse LT94 and capelin oil from southern hemisphere supplied by Sopropêche.
‡ Supplied by Sud-Ouest Aliment.
§ Vegetable oils (% diet): 4·2 % rapeseed oil; 4·2 % linseed oil; 2·1 % palm oil (Daudry).
∥ Supplied by Louis François.
¶ Attractant mixture (g/kg diet): glucosamine, 5; taurine, 3; betaine, 3; glycine, 2; alanine, 2.
** Vitamin mixture (per kg diet): retinol acetate, 1·5 mg; cholecalciferol, 62·5 μg; dl-α-tocopherol acetate, 50 mg; sodium menadione bisulphate, 10 mg; thiamine–HCl, 1 mg; riboflavin, 4 mg; niacin, 10 mg; d-calcium panthothenate, 20 mg; pyridoxine–HCl, 3 mg; meso-inositol, 300 mg; d-biotin, 0·2 mg; folic acid, 1 mg; cyanocobalamin, 10 μg; l-ascorbyl-2-polyphosphate 50 mg; choline, 1 g. All ingredients were diluted with α-cellulose.
†† Mineral mixture (per kg diet): CaHPO4.2H2O, 5 g; CaCO3, 2·15 g; NaCl, 0·4 g; FeSO4.7H2O, 0·2 g; MnSO4.H2O, 30 mg; ZnSO4.7H2O, 40 mg; CuSO4.5H2O, 30 mg; CoCl2.6H2O, 0·2 mg; KI, 0·4 mg; NaF, 10 mg; MgOH, 1·24 g; KCl, 0·9 g. All ingredients were diluted with α-cellulose.
‡‡ Se-enriched yeast Selsaf containing 2·2 g Se/kg with 97–99 % of organic Se supplied by Lesaffre.
Fish
Eyed-embryos of all-female diploid rainbow trout (O. mykiss) were provided by Viviers de Sarrance to the INRA experimental fish farm in Donzacq (Landes, France). Rainbow trout fry were randomly allocated to eighteen fibreglass tanks (50 litres) supplied with flow-through spring water at 17°C with 200 fish/tank. From the swim-up stage, which corresponds to the beginning of exogenous feeding, fish (initial body weight: 91 mg) were hand-fed six times a day to apparent satiation. Each diet was distributed to three replicate groups of fish over a 12-week growth trial. For sampling at the start and the end of the feeding trial, fish were feed-deprived for 16 h, killed with an overdose of benzocaine, weighed, frozen in liquid N2 and stored at − 80°C before analysis. All experimental procedures complied with the European Directive 010/63/EU for the protection of animals used for scientific purposes, and the French Decree no. 2001-464 for animal experimentation.
Determination of proximate, mineral and fatty acid composition
Proximate composition of diets and whole fish was determined according to the following procedures: DM after drying at 105°C for 24 h, protein (N × 6·25) by the Kjeldahl method after acid digestion( 27 ), ash by incineration at 550°C for 10 h and gross energy in an adiabatic bomb calorimeter. Total lipid was extracted and measured gravimetrically according to Folch et al. ( Reference Folch, Lees and Sloane Stanley 28 ) using dichloromethane instead of chloroform. Total P was determined by the molybdateblue/ascorbic acid method at 820 nm after mineralisation and acid digestion( 29 ). For total Se determination, diet and whole fry samples were first subjected to acid digestion using concentrated HNO3 and H2O2, samples were then diluted with water until a concentration of 2 % HNO3 was reached, and total Se concentration was measured in the digesta by inductively coupled plasma MS (Agilent 7500ce; Yokogawa Analytical Systems)( Reference Pedrero, Murillo and Camara 30 ). The instrument was equipped with a collision/reaction cell, and H2 was used as reaction gas in order to remove interferences from argon dimers. Matrix effects were corrected by the use of internal calibration, and Ga was used as internal standard. Fatty acid methyl esters were prepared and analysed as described previously( Reference Fontagné, Lataillade and Brèque 31 ). The concentration of the individual fatty acid was expressed in percentage of total fatty acids.
Determination of oxidative status
Peroxide value of total lipid from diets or whole fish was assessed by colorimetric determination of iron-thiocyanate according to Shantha & Decker( Reference Shantha and Decker 32 ). Conjugated dienes (E232) and trienes (E268) were measured as specific extinctions at the wavelengths of 232 and 268 nm, respectively( 33 ). Anisidine value was determined according to standard procedures( 34 ). For measurement of lipid-soluble fluorescent products, fluorescence intensity of 10 mg total lipid diluted in 1 ml chloroform–methanol (7:3, v/v) was determined in a spectrofluorometer (Triad Dynex, Serlabo), using excitation/emission wavelengths of 360/465 nm, and quinine sulphate as standard at a concentration of 1 μg/ml in 0·05 m-sulphuric acid( Reference Hammer and Braum 35 , Reference Mourente and Diaz-Salvago 36 ). Oxidised glutathione (GSSG) and reduced glutathione (GSH) were measured in whole fish homogenates using Cayman glutathione assay kit (Bertin Pharma) according to the manufacturer's instructions.
Determination of antioxidant enzyme activity
Antioxidant enzyme activities were assayed in whole fish as described previously( Reference Fontagné-Dicharry, Lataillade and Surget 37 ). Briefly, superoxide dismutase (SOD, EC 1.15.1.1) activity was measured by monitoring the inhibition of nitrotetrazolium reduction at 550 nm, and was expressed as the amount of enzyme required to inhibit the rate of nitrotetrazolium reduction by 50 %/mg protein at 37°C. Catalase (CAT, EC 1.11.1.6) activity was measured by monitoring the decomposition of H2O2 at 240 nm, and was expressed as μmol H2O2 reduced/min per mg protein at 30°C. GPX (EC 1.11.1.9) activity was assayed by the coupled reaction with glutathione reductase (GR) using cumene hydroperoxide and H2O2 as substrates for measuring total GPX and Se-dependent GPX (Se-GPX) respectively, and was expressed as nmol NADPH oxidised/min per mg protein at 30°C. GR (EC 1.6.4.2) activity was determined by monitoring NADPH oxidation at 340 nm, and was expressed as nmol NADPH oxidised/min per mg protein at 30°C. Glutathione-S-transferase (GST, EC 2.5.1.18) activity was assayed by following the conjugation of glutathione with 1-chloro-2,4-dinitrobenzene at 340 nm, and was expressed as nmol S-2,4-dinitrophenylglutathione formed/min per mg protein at 30°C. TR (EC 1.8.1.9) activity was determined by monitoring the reduction of 2-nitrobenzoic acid with NADPH to 5-thio-2-nitrobenzoic acid at 412 nm, and was expressed as increase in absorbance at 412 nm/min per mg protein at pH 7·0 at 25°C. Protein concentration was determined by the method of Lowry et al. ( Reference Lowry, Rosebrough and Farr 38 ) using bovine serum albumin as a standard.
Determination of antioxidant enzyme expression
Total RNA was isolated from whole fish using Trizol reagent (Invitrogen). For quantitative RT-PCR, complementary DNA was generated from 1 μg total RNA using SuperScript® III RT (Invitrogen) and a mix of oligo(dT)15 and random primers (Promega). For each sample, RT was performed in duplicate and quantitative PCR analyses were performed in duplicate using the iQTM SYBR® Green Supermix (Bio-Rad) in a total volume of 15 μl containing 5 μl of the diluted RT reaction mixture (dilution 64) and 200 nm of each primer (Table 2) as described previously( Reference Daprà, Geurden and Corraze 39 ).
Table 2 Oligonucleotide primers used to assay gene expression by quantitative RT-PCR

GPX, glutathione peroxidase; SelP, selenoprotein P; MSRB, methionine-R-sulphoxide reductase B; TR, thioredoxin reductase; SOD, superoxide dismutase; CAT, catalase; GR, glutathione reductase; GST, glutathione-S-transferase; Gclc, glutamate–cysteine ligase catalytic subunit; Nrf2, nuclear factor erythroid-2-related factor 2.
* As described by Pacitti et al ( Reference Pacitti, Wang and Page 6 ).
Statistical analyses
Results are shown as the means with their standard errors. Differences between dietary groups were evaluated using two-way ANOVA followed by Student–Newman–Keuls post hoc analysis to test the effects of Se supplementation and dietary basis. As interaction between Se supplementation and dietary bases (P or F) was found to be significant for some parameters, differences between dietary groups are shown using one-way ANOVA and the main results of two-way ANOVA are presented in Table 3. Percentage data were arc-sin transformed, and weight data were log-transformed before analysis. For gene expression analysis, relative quantification of the target gene transcript with the β-actin reference gene transcript was performed using the ΔΔC t method( Reference Pfaffl 40 ). Statistical analyses were performed with the computing program Statbox (Grimmer Logiciels) and results with P< 0·05 were considered significant.
Table 3 Growth performance, final whole-body composition, glutathione content, antioxidant enzyme activity and gene expression of rainbow trout fry fed the experimental diets for 12 weeks (Mean values with their standard errors; n 6 tanks per selenium supplementation and n 9 tanks per dietary basis)

Se0, diets not supplemented with Se; SeS, diets supplemented with sodium selenite; SeY, diets supplemented with Se-enriched yeast; P, plant-based diets; F, fishmeal-based diets; GSH, reduced glutathione; GSSG, oxidised glutathione; GPX, glutathione peroxidase; Se-GPX, Se-dependent glutathione peroxidase; CAT, catalase; Gclc, glutamate–cysteine ligase catalytic subunit; Nrf2, nuclear factor erythroid-2-related factor 2.
a,b,cMean values within rows and for each diet-related effect (Se supplementation or dietary basis) with unlike superscript letters were significantly different (P< 0·05; two-way ANOVA and Student–Newman–Keuls post hoc test).
* Equivalent SI unit for mU/mg protein for total GPX and Se-GPX is pmol NADPH oxidised/min per mg protein at 30°C.
† Equivalent SI unit for U/mg protein for CAT is μmol H2O2 reduced/min per mg protein.
Results
Growth performance and whole-body composition
Rainbow trout fry readily accepted the experimental diets from the beginning of the investigation, and maintained normal behaviour throughout the growth trial. As the fish were fed to excess or visual satiety, feed intake and feed efficiency were not calculated, but these parameters were evaluated to check that no group was underfed. The survival remained high (>94 %) by day 15 for all dietary treatments. From day 67 onwards, survival was significantly lower in fish fed F-diets than in fish fed P-diets (87 v. 91 %), but was not significantly affected by dietary Se sources and levels (Table 3). Mean wet weight increased approximately ninety-fold during the 12-week feeding trial and no significant differences were recorded between dietary groups (Table 3).
Final whole-body composition was significantly affected by dietary Se supplementation and dietary basis according to two-way ANOVA with the exception of crude protein (Table 3). Whole-body lipid content increased from 4·2 to 10·5 % during the feeding trial. In relation to diet composition, fish fed P-diets displayed higher DM and lipid content and lower ash and P content than fish fed F-diets (Table 3). Higher DM and lipid contents and lower ash content were also noticed in fish fed Se-supplemented diets SeS and SeY, irrespective of dietary basis with plant ingredients (P) or fishmeal (F).
Higher total Se contents were observed in fish fed F-diets and Se-supplemented diets SeS and SeY than in fish fed P-diets and non-supplemented diets Se0, respectively (Fig. 1). Whole-body total Se increased in fish fed Se-enriched yeast (SeY) compared to sodium selenite (SeS), irrespective of the basal diet. Compared to initial first-feeding swim-up fry, the Se concentration was reduced in whole-body of rainbow trout fry fed the plant-based diets PSe0 and PSeS.

Fig. 1 Total selenium content in initial (![]() ) and final (
) and final (![]() ) whole-body of rainbow trout fry fed the plant-based diet not supplemented with selenium (PSe0), the plant-based diet supplemented with sodium selenite (PSeS), the plant-based diet supplemented with selenium-enriched yeast (PSeY), the fishmeal-based diet not supplemented with selenium (FSe0), the fishmeal-based diet supplemented with sodium selenite (FSeS) or the fishmeal-based diet supplemented with selenium-enriched yeast (FSeY) for 12 weeks. Values are means, with their standard errors represented by bars (n 3 tanks per group). a,b,c,d,e,fMean values with unlike letters were significantly different (P< 0·05; one-way ANOVA and Student–Newman–Keuls post hoc test).
) whole-body of rainbow trout fry fed the plant-based diet not supplemented with selenium (PSe0), the plant-based diet supplemented with sodium selenite (PSeS), the plant-based diet supplemented with selenium-enriched yeast (PSeY), the fishmeal-based diet not supplemented with selenium (FSe0), the fishmeal-based diet supplemented with sodium selenite (FSeS) or the fishmeal-based diet supplemented with selenium-enriched yeast (FSeY) for 12 weeks. Values are means, with their standard errors represented by bars (n 3 tanks per group). a,b,c,d,e,fMean values with unlike letters were significantly different (P< 0·05; one-way ANOVA and Student–Newman–Keuls post hoc test).
Fatty acid composition and lipid peroxidation
The fatty acid profile of whole rainbow trout fry was significantly modified by dietary basis (Table 4). The changes in fatty acid composition of fry reflected dietary fatty acid composition with lower levels of SFA and n-3 PUFA, especially n-3 long-chain PUFA (18 v. 25, 20 v. 29 and 0 v. 24 % respectively in P-diets and F-diets) and higher levels of MUFA and n-6 PUFA (38 v. 33 and 22 v. 4 % respectively) in P-diets, compared to F-diets. The fatty acid profile of whole fish was not significantly affected by dietary Se sources and levels with two exceptions (Table 4). A higher n-6 PUFA content (due to a higher 18 : 2n-6 content) was noticed in fish fed the non-supplemented PSe0 diet compared to other Se-supplemented P-diets PSeS and PSeY, and a lower n-3 PUFA content (due to lower 20 : 4n-3, 22 : 5n-3 and 22 : 6n-3 contents) was found in fish fed the non-supplemented FSe0 diet, compared to other Se-supplemented F-diets FSeS and FSeY.
Table 4 Fatty acid profile (% total fatty acids) and oxidative status of total lipid from whole rainbow trout fry fed the experimental diets for 12 weeks (Mean values with their standard errors; n 3 tanks per diet)

PSe0, plant-based diet not supplemented with Se; PSeS, plant-based diet supplemented with sodium selenite; PSeY, plant-based diet supplemented with Se-enriched yeast; FSe0, fishmeal-based diet not supplemented with Se; FSeS, fishmeal-based diet supplemented with sodium selenite; FSeY, fishmeal-based diet supplemented with Se-enriched yeast; PV, peroxide value expressed as milliequivalents of active oxygen per kg total lipid; E232, conjugated dienes measured as specific extinction at the wavelength of 232 nm; E268, conjugated trienes measured as specific extinction at the wavelength of 268 nm; AV, anisidine value; LSFP, lipid-soluble fluorescent products.
a,b,c,dMean values within a row with unlike superscript letters were significantly different (P< 0·05; one-way ANOVA and Student–Newman–Keuls post hoc test).
Some biomarkers of lipid peroxidation such as E232 and anisidine value assessed in total lipid of whole rainbow trout fry were reduced in fish fed P-diets, compared to fish fed F-diets in relation with the oxidative status of diets (16·0 and 2·5 v. 20·5 and 3·0 respectively in P-diets and F-diets). However, the oxidative status of fish was not significantly affected by dietary Se sources and levels (Table 4). On the other hand, GSSG content was significantly higher in fish fed P-diets, compared to fish fed F-diets, and the GSH:GSSG ratio was significantly higher in fish fed SeY-diets, compared to fish fed Se0 or SeS-diets according to two-way ANOVA (Table 3).
Antioxidant enzyme activity and gene expression
The whole-body Se-GPX activity was significantly lower in rainbow trout fry fed the non-supplemented plant-based diet PSe0 (Fig. 2). According to two-way ANOVA, whole-body Se-GPX activity was significantly higher in fish fed Se-yeast (diets SeY), compared to fish fed sodium selenite (diets SeS) or non-supplemented diets Se0 (Table 3). The activity of other antioxidant enzymes was not significantly affected by dietary Se sources and levels (Table 5). The activity of CAT and total GPX was higher in fish fed F-diets than in fish fed P-diets according to two-way ANOVA (Table 3).

Fig. 2 Activity of selenium-dependent glutathione peroxidase (Se-GPX) in initial (![]() ) and final (
) and final (![]() ) whole-body of rainbow trout fry fed the plant-based diet not supplemented with selenium (PSe0), the plant-based diet supplemented with sodium selenite (PSeS), the plant-based diet supplemented with selenium-enriched yeast (PSeY), the fishmeal-based diet not supplemented with selenium (FSe0), the fishmeal-based diet supplemented with sodium selenite (FSeS) or the fishmeal-based diet supplemented with selenium-enriched yeast (FSeY) for 12 weeks. Values are means, with their standard errors represented by bars (n 3 tanks per group). a,bMean values with unlike letters are significantly different (P< 0·05; one-way ANOVA and Student–Newman–Keuls post hoc test).
) whole-body of rainbow trout fry fed the plant-based diet not supplemented with selenium (PSe0), the plant-based diet supplemented with sodium selenite (PSeS), the plant-based diet supplemented with selenium-enriched yeast (PSeY), the fishmeal-based diet not supplemented with selenium (FSe0), the fishmeal-based diet supplemented with sodium selenite (FSeS) or the fishmeal-based diet supplemented with selenium-enriched yeast (FSeY) for 12 weeks. Values are means, with their standard errors represented by bars (n 3 tanks per group). a,bMean values with unlike letters are significantly different (P< 0·05; one-way ANOVA and Student–Newman–Keuls post hoc test).
Table 5 Antioxidant enzyme activity of whole rainbow trout fry fed the experimental diets for 12 weeks* (Mean values with their standard errors; n 3 tanks per diet)
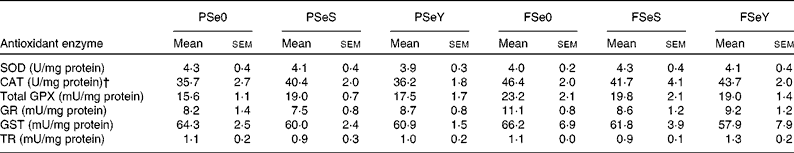
PSe0, plant-based diet not supplemented with Se; PSeS, plant-based diet supplemented with sodium selenite; PSeY, plant-based diet supplemented with Se-enriched yeast; FSe0, fishmeal-based diet not supplemented with Se; FSeS, fishmeal-based diet supplemented with sodium selenite; FSeY, fishmeal-based diet supplemented with Se-enriched yeast; SOD, superoxide dismutase; CAT, catalase; GPX, glutathione peroxidase; GR, glutathione reductase; GST, glutathione-S-transferase; TR, thioredoxin reductase.
*Mean values within any of the rows were non-significantly different (one-way ANOVA; P< 0·05).
† Equivalent SI unit for U/mg protein for CAT is μmol H2O2 reduced/min per mg protein.
Gene expression profiles were correlated to activity levels of antioxidant enzymes with a significant decreased expression of some Se-GPX (GPX1b1, GPX1b2 and GPX4a1) in rainbow trout fry fed the non-supplemented plant-based diet PSe0, and no effect on the expression of genes coding for the other antioxidant enzymes SOD1, SOD2 and GR (Table 6). Expression of GST was reduced in the PSe0 group only, when compared to the FSe0 group. Expression of SelP was significantly reduced in the PSe0 group, whereas gene expression profiles of the other selenoproteins GPX1a, GPX4a2, GPX4b and TR1 were not affected by dietary Se sources and levels. Expression of the selenoprotein MSRB2 was the lowest in the PSe0 group and the highest in the FSe0 group. On the other hand, according to two-way ANOVA, gene expression of CAT was higher in fish fed F-diets, compared to fish fed P-diets, and gene expressions of CAT, glutamate–cysteine ligase catalytic subunit (Gclc) and redox- and electrophile-sensitive transcription factor nuclear factor erythroid-2-related factor 2 (Nrf2) were higher in fish fed Se0 and SeS-diets, compared to fish fed SeY-diets (Table 3).
Table 6 Antioxidant enzyme expression of whole rainbow trout fry fed the experimental diets for 12 weeks* (Mean values with their standard errors; n 3 tanks per diet)

PSe0, plant-based diet not supplemented with Se; PSeS, plant-based diet supplemented with sodium selenite; PSeY, plant-based diet supplemented with Se-enriched yeast; FSe0, fishmeal-based diet not supplemented with Se; FSeS, fishmeal-based diet supplemented with sodium selenite; FSeY, fishmeal-based diet supplemented with Se-enriched yeast; GPX, glutathione peroxidase; SelP, selenoprotein P; MSRB, methionine-R-sulphoxide reductase B; TR, thioredoxin reductase; SOD, superoxide dismutase; CAT, catalase; GR, glutathione reductase; GST, glutathione-S-transferase, Gclc, glutamate–cysteine ligase catalytic subunit; Nrf2, nuclear factor erythroid-2-related factor 2.
a,b,c,dMean values within a row with unlike superscript letters are significantly different (P< 0·05; one-way ANOVA and Student–Newman–Keuls post hoc test).
* Transcript expression was normalised to β-actin RNA and values are expressed as a ratio of the FSe0 group.
Discussion
Growth performance of rainbow trout fry was not affected by dietary Se sources and levels ranging from 0·5 to 1·7 mg/kg diet in accordance with the results reported in juvenile rainbow trout by Hilton et al. ( Reference Hilton, Hodson and Slinger 41 ), who estimated threshold levels at 0·15 mg/kg diet for requirement and 3 mg/kg diet for toxicity. Although there was a slight reduction in survival of rainbow trout fry fed fishmeal-based diets with the highest dietary Se content (from 1·2 to 1·7 mg/kg), no mortality in relation to dietary Se deficiency was recorded as reported for salmon fry( Reference Poston, Combs and Leibovitz 42 ). Growth performance was not higher in fish fed F-diets than in fish fed P-diets, as observed in rainbow trout juveniles( Reference Antony Jesu Prabhu, Schrama and Mariojouls 43 ). High dietary Se has been shown to exert toxic effects such as elevated mortality, reduced growth and feed efficiency as well as renal calcinosis at levels marginally above those required( 13 ), but the low survival of fish fed F-diets is probably more indicative of another dietary mineral deficiency. Indeed, the F-diets were not supplemented with mineral mixture, whereas the P-diets contained 1 % mineral mixture. Fish meal-based diets contained only 10 mg Mn/kg diet, slightly lower than the estimated requirement level of 12–13 mg/kg( 13 ), whereas the plant-based diets contained 80 mg/kg( Reference Antony Jesu Prabhu, Schrama and Mariojouls 43 ). A fishmeal-based diet without Mn supplement has been shown to cause poor hatchability in rainbow trout broodstock( Reference Takeuchi, Watanabe and Ogino 44 ).
Uptake of Se by fish can be from water or diet( Reference Watanabe, Kiron and Satoh 16 , Reference Hamilton 45 ). The waterborne Se content of 2·2 μg/l determined at the beginning of the present study according to Darrouzès et al. ( Reference Darrouzès, Bueno and Simon 46 , Reference Darrouzès, Bueno and Lespes 47 ) was within the range that rarely produced discernible adverse effects on fish according to Hamilton( Reference Hamilton 45 ). This level was also unlikely to contribute significantly to the dietary requirement for Se even if waterborne Se has been shown to be readily accumulated by rainbow trout( Reference Hodson, Hilton and Slinger 48 ). The lowest whole-body Se content was found in fish fed the non-supplemented plant-based diet PSe0. The whole-body Se concentration of these fish was particularly low, as it was lower compared to initial fish from the present study or rainbow trout juveniles fed a similar diet( Reference Antony Jesu Prabhu, Schrama and Mariojouls 43 ). A reduced tissue Se concentration associated with a low GPX activity has been reported in rainbow trout fed Se-deficient diet( Reference Bell, Cowey and Adron 17 , Reference Hilton, Hodson and Slinger 41 , Reference Bell, Pirie and Adron 49 – Reference Küçükbay, Yazlak and Karaca 51 ) and in other fish species( Reference Wang, Han and Li 52 – Reference Penglase, Hamre and Rasinger 58 ). However, the total Se accumulation in fish tissues (except for kidney) has been shown to be dose-dependent( Reference Lee, Lee and Bai 59 ), and thus a reduced whole-body Se content is indicative of a reduced dietary Se level, but not necessarily of a Se-deficient diet.
A dietary Se deficiency has generally been reported to result in growth reduction as well as reduced activity of GPX( 13 ). Hepatic GPX activity has been found to be a more stringent and more robust criterion than weight gain to define Se deficiency( Reference Antony Jesu Prabhu, Schrama and Kaushik 60 ). In the present study, due to the small size of fish, Se-GPX activity was determined in the whole-body of rainbow trout fry and not in liver. A lower whole-body Se-GPX activity was noticed in rainbow trout fry fed the non-supplemented plant-based diet PSe0, suggesting a dietary Se deficiency in this group of rainbow trout fry fed 0·5 mg Se/kg diet. This result also suggests the possibility of using whole-body Se-GPX activity to define Se deficiency in small fish. Based on plasma GPX activity and using purified diets supplemented with sodium selenite, Hilton et al. ( Reference Hilton, Hodson and Slinger 41 ) defined the Se requirement at 0·38 mg/kg for rainbow trout juveniles. The Se requirement for fish is known to be affected by the type of Se source( Reference Antony Jesu Prabhu, Schrama and Kaushik 60 ). Organic sources such as selenomethionine or Se-enriched yeast but not selenocysteine have been demonstrated to be more available than inorganic sources such as sodium selenite( Reference Antony Jesu Prabhu, Schrama and Kaushik 60 ). Se availability from plant-derived proteins is reported to be considerably higher than that from fishmeals( Reference Watanabe, Kiron and Satoh 16 ). Wheat and soyabean used to formulate P-diets are supposed to contain predominantly selenomethionine with lesser amounts of selenocysteine and selenate( Reference Rayman, Infante and Sargent 23 ). So the higher Se requirement of fish from the present study compared to the study of Hilton et al. ( Reference Hilton, Hodson and Slinger 41 ) might not be attributed to a lower Se availability from the PSe0 diet compared to sodium selenite. The Se requirement for fish is also known to be affected by interaction between dietary vitamin E and Se( 13 , Reference Antony Jesu Prabhu, Schrama and Kaushik 60 ). However, the dietary vitamin E requirement of rainbow trout was assumed to be fulfilled in the present study with the supplementation of 50 mg α-tocopherol acetate/kg diet( 13 ). On the other hand, Bell et al. ( Reference Bell, Cowey and Adron 17 ) suggested a higher dietary Se requirement in early life stages to explain the absence of pathology in rainbow trout juveniles fed low dietary Se levels in their study compared to the only available study carried out with early developmental stages of fish by Poston et al. ( Reference Poston, Combs and Leibovitz 42 ). The early developmental stages that are characterised by a high growth rate are also more susceptible to oxidative stress than the juvenile stage, possibly due to delayed response or lack of complete development of endogenous antioxidant defence system, and so rainbow trout may need more dietary antioxidant micronutrients at the beginning of their life( Reference Fontagné, Lataillade and Brèque 31 , Reference Fontagné-Dicharry, Lataillade and Surget 37 ). Recent studies have shown beneficial effects of high dietary Se supplementation (between 3 and 5 mg Se/kg) for fish larvae( Reference Ribeiro, Ribeiro and Sæle 55 , Reference Penglase, Nordgreen and van der Meeren 61 , Reference Betancor, Caballero and Terova 62 ), whereas the tested levels were similar to the threshold levels defined for adverse effects in fish juveniles( 13 ), suggesting differences in Se metabolism of fish larvae and of juveniles.
The reduction of whole-body Se-GPX activity in the PSe0 group did not lead to an increased compensatory whole-body GST activity contrary to findings in liver and plasma of juvenile rainbow trout or channel catfish( Reference Gatlin, Poe and Wilson 15 , Reference Bell, Pirie and Adron 49 , Reference Bell, Adron and Cowey 50 ). However, as total GPX was not significantly different between groups, an induction of the non-Se-dependent GPX can be assumed. The reduced activity of Se-GPX in fish fed PSe0 was associated with concomitant decreased expression of the Se-GPX gene isoforms GPX1b1, GPX1b2 and GPX4a1 confirming the observations on GPX transcript expression in rainbow trout hepatocytes( Reference Pacitti, Wang and Page 6 ), zebrafish( Reference Penglase, Hamre and Rasinger 58 ), cod larvae( Reference Penglase, Nordgreen and van der Meeren 61 ) and rats( Reference Barnes, Evenson and Raines 63 ). In contrast, GPX1a did not show any significant difference in expression level contrary to what has been reported in vitro with GPX1a being more induced by Se at low/intermediate concentrations, and GPX1b1 and GPX1b2 at high concentrations( Reference Pacitti, Wang and Page 6 ). GPX4 appeared to be less sensitive to Se exposure with GPX4a1 being the most sensitive isoform in rainbow trout hepatocytes( Reference Pacitti, Wang and Page 6 ), whereas the lack of regulation of GPX4 by Se has been reported in cod larvae( Reference Penglase, Nordgreen and van der Meeren 61 ) and mammals( Reference Brigelius-Flohé 3 , Reference Barnes, Evenson and Raines 63 , Reference Zhou, Zhao and Li 64 ).
Other selenoproteins such as TR were not affected by dietary Se sources and levels, except for SelP whose expression was lower in fish fed the PSe0 diet. These results seem to be in contradiction to the two studies in rainbow trout juveniles fed fishmeal-based diets where a higher hepatic TR activity was noticed in fish fed diets supplemented with the highest level of Se-enriched yeast( Reference Rider, Davies and Jha 19 , Reference Rider, Davies and Jha 65 ). This is possibly due to the fact that the dietary Se levels tested were different between the three studies: from 0·5 to 1·7 mg/kg diet in the present study as against 0·7–7·4 in the studies of Rider et al. ( Reference Rider, Davies and Jha 19 , Reference Rider, Davies and Jha 65 ). As the Se levels tested in the latter studies were above requirements, GPX activity was not affected( Reference Rider, Davies and Jha 19 , Reference Rider, Davies and Jha 65 ). The dietary Se levels tested in the present study might not be high enough to observe an impact on whole-body TR activity and gene expression. A hierarchy of selenoprotein expression has been recognised in mammals when Se is limiting with a preferential expression of essential selenoproteins such as TR1 compared to stress-related selenoproteins such as GPX1 and SelP( Reference Mariotti, Ridge and Zhang 12 , Reference Schomburg and Schweizer 66 ) in accordance with the results of the present study.
The genes of some antioxidant enzymes (GST, glutamate–cysteine ligase, SOD1) and the selenoprotein TR1 contain the antioxidant response element in their promoter regions, and can thus be induced by activation of the transcription factor Nrf2( Reference Kwak, Wakabayashi and Kensler 67 ). Different mechanisms appear to be involved in Nrf2 activation, including both post-transcriptional and transcriptional events, as an increase in Nrf2 gene transcription has been reported in certain types of cells or physiological conditions( Reference Kwak, Wakabayashi and Kensler 67 ). In the present study, GST, SOD1 and TR1 mRNA levels were not affected by dietary Se sources and levels in contrast to the results of Burk et al. ( Reference Burk, Hill and Nakayama 68 ) who reported an induction of GST by dietary Se deficiency through Nrf2-antioxidant response element in mouse liver. We found that Nrf2 was induced in fish fed the non-supplemented diets Se0, compared to fish fed Se-enriched yeast, but was repressed compared to fish fed sodium selenite. Only glutamate–cysteine ligase was induced in fish fed the FSe0-diet, compared to fish fed FSeS or FSeY-diets. This discrepancy could be related to a difference in dietary Se levels in mouse fed a Se-deficient diet containing less than 0·01 mg Se/kg v. 0·5 mg Se/kg in the present study, which was possibly not deficient enough to observe an impact on the Nrf2-antioxidant response element pathway. However, it could also be related to a difference in the level of induced oxidative stress in animals fed non supplemented Se-diets, since we observe a higher Nrf2 and Gclc gene expression in trout fed the FSe0 diet, compared to those fed the FSeY diet. Fish fed F-diets were characterised by a higher lipid peroxidation level than fish fed P-diets, which might be necessary to promote the activation of Nrf2.
The lipid peroxidation status was higher in lipids of rainbow trout fry fed F-diets, but was not significantly affected by dietary Se sources and levels. These results are consistent with previous observations in fingerling channel catfish( Reference Gatlin, Poe and Wilson 15 ) and common carp fed 0 or 1·5 mg/kg of diphenyl diselenide( Reference Menezes, Leitemperger and Santi 69 ). However, dietary Se supplementation has been shown to reduce lipid peroxidation in juvenile rainbow trout( Reference Bell, Cowey and Adron 17 , Reference Küçükbay, Yazlak and Karaca 51 ), sea bass larvae( Reference Betancor, Caballero and Terova 62 ) and common carp fed 0 or 3 mg/kg of diphenyl diselenide( Reference Menezes, Leitemperger and Santi 69 ). Differences could be due to the tested dietary Se levels, since the antioxidant effect of Se was seen in common carp at 3 mg/kg but not at 1·5 mg/kg( Reference Menezes, Leitemperger and Santi 69 ), and Se has also been shown to be a prooxidant at high levels in several fish species( Reference Rider, Davies and Jha 65 ) such as 5 mg/kg for common carp( Reference Menezes, Leitemperger and Santi 69 ). Differences could also be due to the form of Se and the sensitivity of the marker used to assess lipid peroxidation, since the GSH:GSSG ratio was higher in rainbow trout fry fed Se-yeast than in fish fed sodium selenite or non-supplemented diets, contrary to the other markers that were not significantly affected by dietary Se sources and levels. The lack of effect of supplemental Se on these markers of lipid peroxidation may also indicate that the decrease of the antioxidant status noticed in fish fed the non-supplemented plant-based diet PSe0 is not critical enough to provoke oxidative stress in the low stressful conditions used in the present study (e.g. low stocking density), as also reported by the observations of Küçükbay et al. ( Reference Küçükbay, Yazlak and Karaca 51 ).
Present results highlight the influence of the dietary Se form on whole-body Se content and activity of GPX in rainbow trout fry, confirming data on the greater bioavailability of organic Se compared to inorganic sources( Reference Jaramillo, Peng and Gatlin DM 18 , Reference Rider, Davies and Jha 19 , Reference Küçükbay, Yazlak and Karaca 51 , Reference Antony Jesu Prabhu, Schrama and Kaushik 60 ). Moreover, a higher GSH:GSSG ratio and a decreased CAT, glutamate–cysteine ligase and Nrf2 gene expression indicative of decreased oxidative stress was noticed in rainbow trout fry fed Se-yeast compared to sodium selenite, confirming the antioxidant properties of some selenoproteins that were affected by the organic Se levels tested in the present study.
In conclusion, the present work indicates that feeding rainbow trout fry with plant-based diets without Se supplementation might be detrimental to the antioxidant status of fish. It also confirms the superiority of organic forms to fulfil the dietary Se requirement of rainbow trout fry. It also supports the observations that whole-body GPX and SelP expression are good markers of Se deficiency in rainbow trout fry.
Acknowledgements
The authors wish to thank F. Terrier, F. Sandres and Y. Hontang for the preparation of diets and care of fish. They are also very grateful to V. Véron, L. Larroquet, M. Cluzeaud, A. Surget and M.-J. Borthaire for technical assistance.
The present study was supported by the INRA-WUR aquaculture platform (P. A. J. P., PhD fellowship), China Scholarship Council (H. L., grant no. 201204910354) and Conseil Régional d'Aquitaine (S. G., B. B. and M. B., grant no. 20071303002PFM).
The authors' contributions are as follows: S. F.-D., P. A. J. P., P. T., F. M. and S. J. K. designed the study; S. G. and H. L. conducted the study; S. F.-D., B. B. and M. B. analysed the data; S. F.-D., S. G. and S. J. K. wrote the article; S. F.-D. had primary responsibility for the final content. All authors read and approved the final manuscript. The authors declare that there are no conflicts of interest.













