Cows' milk (CM) proteins are the most frequent cause of food allergy during infancy. Depending on diagnostic criteria and study design, estimates of the prevalence of cows' milk protein allergy (CMPA) vary from 2 to 7·5 %( Reference Vandenplas, Koletzko and Isolauri 1 ).
The first-line treatment for food allergy disorders is avoidance of the suspected allergen. In the particular case of CMPA, guidelines recommend the use of formulas in which CM proteins are extensively hydrolysed( Reference Vandenplas, Koletzko and Isolauri 1 , Reference Koletzko, Niggemann and Arato 2 ). By reducing the number of conformational and sequential epitopes, extensive hydrolysis dramatically reduces allergenicity of CM proteins. This avoidance of contact to allergens is the primary objective of using extensively hydrolysed formulas and most often allows infants to thrive while progressively outgrowing CMPA. Thus, hypo-allergenic formulas should ensure a normal development of the infant; however, data relating to the impact of these formulas on infants' growth are insufficient( Reference Agostoni, Fiocchi and Riva 3 – Reference Savino, Castagno and Monti 5 ).
In 2008, the Cow's Milk Allergy Modified by Elimination and Lactobacilli (CAMEL) study was a randomised, double-blind, placebo-controlled trial, funded by the Dutch Ministry of Economic Affairs, that aimed at determining whether acquisition of tolerance to CM would be affected by supplementation to the infant formula with a combination of two probiotics (Lactobacillus casei CRL431 and Bifidobacterium lactis Bb-12)( Reference Hol, van Leer and Elink Schuurman 6 ). This study included 119 allergic infants fed with an extensively hydrolysed casein-based formula (eHCF) either supplemented with probiotics or without probiotics for 6 months. The probiotic supplementation did not improve acquisition of tolerance. However, although collected, the data concerning tolerance of the formula and growth parameters of all infants included in the study did not appear in the initial analysis.
Therefore, the objective of this post hoc analysis was to capitalise on the data pertaining to a population of infants with validated CMPA in order to assess the tolerance/hypo-allergenicity of the formula along with its safety for growth in infants fed this eHCF for 6 months.
Methods
Population and design
The present randomised, double-blind, placebo-controlled study enrolled infants with a diagnosis of CMPA, aged less than 6 months, followed up by the paediatricians of the CAMEL study group. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects were approved by the local ethics committee of the Erasmus MC, Rotterdam. At least, one parent in each family provided written informed consent before inclusion.
Interventions
Details of the procedure have been described previously( Reference Hol, van Leer and Elink Schuurman 6 ). In brief, upon identification, infants with suspected CMPA were prescribed an eHCF for at least 4 weeks (Fig. 1). Following this, CMPA was then confirmed at visit 1 using a food challenge performed according to the current guidelines( Reference Bock, Sampson and Atkins 7 , Reference Bruijnzeel-Koomen, Ortolani and Aas 8 ). All infants with positive challenge were randomised at visit 2 (inclusion visit) to the supplemented or the non-supplemented eHCF group. Skin prick tests against milk, egg and soya were performed at visit 2, and a double-blind, placebo-controlled food challenge (DBPCFC) was performed after 6 months of eHCF feeding (visit 3).

Fig. 1 Design of the study adapted from the Cow's Milk Allergy Modified by Elimination and Lactobacilli study( Reference Hol, van Leer and Elink Schuurman 6 ). CMPA, cows' milk protein allergy; eHCF, extensively hydrolysed casein-based formula; CM, cows' milk; SCORAD, scoring of atopic dermatitis; SPT, skin prick test; DBPCFC, double-blind, placebo-controlled food challenge.
Study products
The eHCF was a commercially available formula especially designed for infants with CMPA (Allernova, Allergy Care; United Pharmaceuticals). The probiotic-supplemented formula used the same eHCF but with L. casei CRL431 and B. lactis Bb-12. Nutritional composition of the eHCF conforms to the essential composition set by the European Directive 1999/21 for foods for special medical purposes and by the European Directive 2006/141 for infant and follow-on formulas, particularly regarding the amino acid profile. The energy content in the formula is 276·9 kJ/100 ml (66·3 kcal/100 ml), and the protein, lipid and carbohydrate contents are 1·6, 3·5 and 7·2 g/100 ml, respectively. The extensive casein hydrolysate has a median peptide size of 267 Da, with more than 95 % of peptides less than 1500 Da.
Measurements
Allergic symptoms were registered after food challenge, at visits 1 and 3. The scoring of atopic dermatitis (SCORAD) index, assessing the severity of the eczema, was measured at visits 2 and 3( 9 ). Weight and length were measured at visits 2 and 3. Weight-for-age, length-for-age, weight-for-length and BMI-for-age z scores were computed for each infant, based on the WHO 2006 reference data( 10 ).
Statistical analyses
Normality of distributions was assessed using the Shapiro–Wilk test. Quantitative parameters were compared within groups between visit 2 (randomisation) and visit 3 using Student's t test (normally distributed data) or Wilcoxon's test (non-normally distributed data), and were compared between groups using ANCOVA based on ranks with the baseline value as a co-variable. Statistical analyses were conducted using SAS version 9.2 (SAS Institute Inc.). Statistical tests were two-sided, and the level of significance was set at 5 %. Sample size calculation showed that ninety-seven patients were required to demonstrate an increase of +0·5 in the weight-for-age z score, considering 1·5 sd and assuming a type-I error of 5 % and a power of 90 %( Reference Agostoni, Fiocchi and Riva 3 ). Distributions of z scores compared with normal reference values were represented using WHO Anthro version 3.2.2 software.
Results
Study population
Of the 193 infants with suspected CMPA referred to the CAMEL study, 119 met the criteria to continue the study following a confirmed CMPA. The mean age was 4·2 months (minimum 1·4, maximum 6·0), and males represented 55 % of included patients. The baseline characteristics of the study population included are summarised in Table 1. At visit 1, the allergic symptoms on oral challenge with a CM formula were urticaria, worsening of atopic eczema/atopic dermatitis (AD) syndrome, vomiting, diarrhoea, physician-diagnosed wheezing or convincing behavioural symptoms. Among the 119 included infants, 54·2 % had skin reactions; 33·9 % had gastrointestinal reactions; 44·1 % had subjective symptoms, such as crying and irritability; and 2·5 % had airway reactions on oral challenge at visit 1 (Table 2). Among infants who had subjective symptoms, 32·7 % expressed also skin symptoms, 36·5 % gastrointestinal symptoms and 1·9 % airway reactions; 23·1 % had two or more other symptoms than subjective symptoms, and 9·6 % expressed symptoms in two organ systems.
Table 1 Baseline characteristics of included infants (Mean values and standard deviations; number of subjects and percentages)
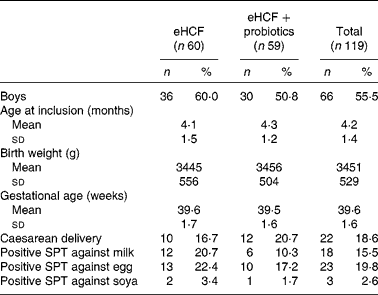
eHCF, extensively hydrolysed casein-based formula; SPT, skin prick test.
Table 2 Symptoms that developed during oral food challenge at visit 1 by allergic infants (Number of subjects and percentages)
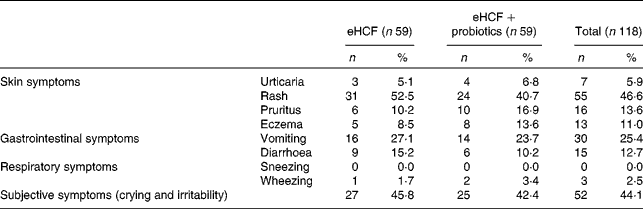
eHCF, extensively hydrolysed casein-based formula.
Hypo-allergenicity/tolerance
All the 193 screened infants were fed the eHCF for at least 4 weeks or more. All of them clinically tolerated the eHCF well, including the 119 infants who had an overt CMPA and could eventually be maintained in the follow-up, 6-month study.
During the following 6 months, eight subjects dropped out: five lost to follow-up; two consents retracted by the parents; one study formula stopped by the paediatrician. Of the 111 included infants who completed the 6-month feeding period, sixty-one became tolerant to CM and fifty were still allergic to CM at 6 months according to a DBPCFC.
At visit 3, among the fifty infants still allergic to CM, 59·2 % had skin reactions, 36·7 % gastrointestinal reactions, 51·0 % subjective symptoms and 4·1 % airway reactions after the DBPCFC. Details of each item are described in Table 3. Among the infants who had subjective symptoms, 27·8 % expressed also skin symptoms, 77·8 % gastrointestinal symptoms and 5·6 % airway reactions; 27·8 % had two or more other symptoms than subjective symptoms, and 27·8 % expressed symptoms in two organ systems.
Table 3 Symptoms that developed during double-blind, placebo-controlled food challenge at 6 months (visit 3) by still allergic infants (Number of subjects and percentages)
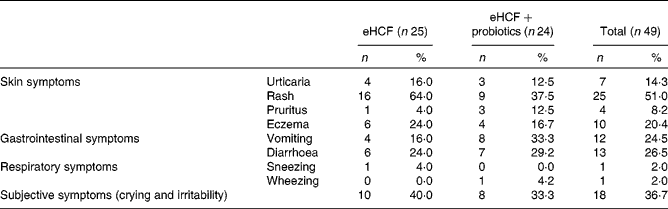
eHCF, extensively hydrolysed casein-based formula.
Evolution of the scoring of atopic dermatitis index at 6 months
The mean SCORAD index of infants fed the eHCF for 6 months significantly improved (Table 4), and decreased from 9·9 (sd 14·2) at randomisation to 5·6 (sd 9·5) at 6 months (P< 0·001, Wilcoxon's test). In the sub-population of infants with eczema (SCORAD>0), the SCORAD index decreased significantly by − 6·6 (sd 13·6; P< 0·001). Probiotic supplementation to the eHCF had, however, no significant effect on the evolution of the SCORAD index.
Table 4 The scoring of atopic dermatitis (SCORAD) index at randomisation and at 6 months (Mean values and standard deviations)
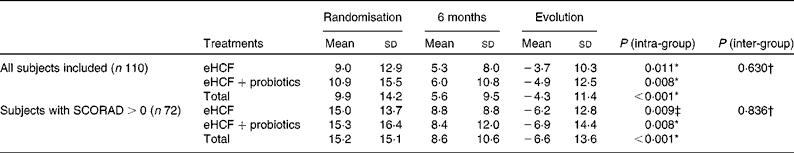
eHCF, extensively hydrolysed casein-based formula.
* Wilcoxon's test.
† ANCOVA based on ranks.
‡ Student's t test.
Anthropometric data
At birth, the mean weight-for-age z scores of included infants were all higher than 0·0 (Table 5). From birth to randomisation in the study, the mean weight-for-age z scores decreased significantly by − 1·5 (sd 1·1) in the entire study population, subgroup analysis showing no difference between the two groups.
Table 5 Weight-for-age z scores at birth and at randomisation (Mean values and standard deviations)

eHCF, extensively hydrolysed casein-based formula.
* Wilcoxon's test.
† ANCOVA based on ranks.
‡ Student's t test.
Following the 6-month feeding of eHCF, a significant improvement was observed for the weight-for-age, length-for-age and weight-for-length z scores, as well as restoration of a normal BMI (Table 6). Subgroup analyses did not show any benefit from the probiotic supplementation. In addition, proportions of infants with length-for-age and weight-for-age z scores below − 2·0 were significantly reduced (Table 7). The distribution of z scores for weight-for-age, length-for-age and weight-for-length at randomisation and after 6 months is shown in Figs. 2–4. At randomisation, the weight-for-age curve followed an expected dispersion but a deviation to the left. The length-for-age curve also showed a deviation to the left but a greater value of dispersion. The weight-for-length curve followed the expected dispersion but showed a slight deviation to the left. A 6-month feeding with the eHCF allowed normalisation of these three distributions.
Table 6 Anthropometric data at randomisation and at 6 months (Mean values and standard deviations)

eHCF, extensively hydrolysed casein-based formula.
* Student's t test.
† ANCOVA.
‡ Wilcoxon's test.
Table 7 Proportions of infants with cows' milk protein allergy and nutritional deficits (z scores <−2·0) (Number of subjects and percentages)
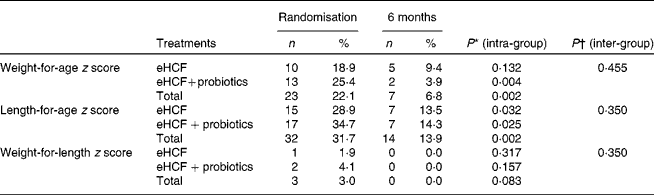
eHCF, extensively hydrolysed casein-based formula.
* McNemar's test.
† Fisher's test.

Fig. 2 Distribution of weight-for-age z scores of all children (![]() ) compared with normal reference values (
) compared with normal reference values (![]() ) at (a) randomisation and (b) after 6 months.
) at (a) randomisation and (b) after 6 months.

Fig. 3 Distribution of length-for-age z scores of all children (![]() ) compared with normal reference values (
) compared with normal reference values (![]() ) at (a) randomisation and (b) after 6 months.
) at (a) randomisation and (b) after 6 months.

Fig. 4 Distribution of weight-for-length z scores of all children (![]() ) compared with normal reference values (
) compared with normal reference values (![]() ) at (a) randomisation and (b) after 6 months.
) at (a) randomisation and (b) after 6 months.
Discussion
This post hoc analysis of the CAMEL study assessed, in a well-characterised population of infants with CMPA proved by oral challenge, the tolerance/hypo-allergenicity of the eHCF and its nutritional adequacy.
A total of 193 infants suspected of having CMPA were fed with the eHCF for at least 4 weeks. All of them, including the 119 infants with CMPA, clinically tolerated the eHCF well for 4 or more weeks, which is more than a sufficient period to detect late-onset allergic reactions according to the American Academy of Paediatrics( 11 ).
Previously published data mainly reported changes in the SCORAD index in infants fed an extensively hydrolysed whey-based formula. Previously, two studies have shown an improvement in this index after 6 and 8 months of extensively hydrolysed whey-based formula treatment( Reference Niggemann, Binder and Dupont 12 , Reference Isolauri, Sütas and Mäkinen-Kiljunen 13 ), as also observed in the present study after 6 months of eHCF feeding. These results are in opposition to those reported by Niggemann et al. ( Reference Niggemann, von Berg and Bollrath 14 ). The SCORAD index results were registered at randomisation and after approximately 28, 60, 90 and 180 d. They did not show any significant improvement and remained constant throughout the trial period. As already evidenced by the CAMEL study group in 2008, the probiotic supplementation had no effect on the evolution of the SCORAD index. Since 2008, Gore et al. ( Reference Gore, Custovic and Tannock 15 ) confirmed the lack of effect that probiotics have on eczema, as they found no benefit from supplementation with B. lactis or Lactobacillus paracasei in the treatment of eczema, in infants aged 3–6 months.
Compared with healthy infants, infants with allergy can have impaired growth, which is partly attributable to improper food substitutions following allergen elimination( Reference Isolauri, Sutas and Salo 16 ). Moreover, CMPA may also increase energy requirements through inflammation (i.e. skin or gastrointestinal) and disrupted sleep, as well as reduce the absorption of major nutrients (i.e. CMPA-induced enteropathy)( Reference Meyer, Venter and Fox 17 ). However, data on the nutritional adequacy of eHCF are insufficient( Reference Dupont, Chouraqui and de Boissieu 4 ). Most of the published growth data were obtained in healthy term infants( Reference Hernell and Lönnerdal 18 – Reference Borschel, Baggs and Barrett-Reis 21 ) or in infants at risk of atopy( Reference Rzehak, Sausenthaler and Koletzko 22 ) and very little in allergic infants, despite the fact that this type of formula is particularly targeted to the latter population. Only three previous trials have reported anthropometric indices in allergic infants fed an eHCF.
The post hoc analysis of growth data obtained in the CAMEL study( Reference Hol, van Leer and Elink Schuurman 6 ) provides interesting data on (1) the length and weight deficit affecting infants with CMPA and (2) the potential catch-up for both length and weight using an eHCF, thereby underlining its safe and nutritional adequacy for infants with CMPA.
Savino et al. ( Reference Savino, Castagno and Monti 5 ) and Agostoni et al. ( Reference Agostoni, Fiocchi and Riva 3 ) showed a decrease in anthropometric indices between birth and inclusion in their study in infants with proved CMPA. In the Savino's study, twenty-six infants fed an eHCF were included at a mean age of 3·33 (sd 2·31) months. The mean weight-for-age z scores of these infants were 0·04 (sd 0·79) at birth and decreased to − 0·39 (sd 0·55) at 2·5 months of age, showing that CMPA induced a reduction in weight gain. Agostoni et al. ( Reference Agostoni, Fiocchi and Riva 3 ) reported that the mean weight-for-age z scores was between − 0·13 and 0·28 at birth, which decreased to − 0·36 to − 0·45 at 6 months of age. The same decrease in weight-for-age z score between birth and study inclusion was observed in the present study. Because of the delay in diagnosis often seen in clinical practice, children with both immediate and delayed-type CMPA are particularly at risk of being undernourished( Reference Niggemann, von Berg and Bollrath 14 ). Isolauri et al. ( Reference Isolauri, Sutas and Salo 16 ) showed that the relative length and weight of infants with CMPA decreased compared with the control group. The decrease in relative length coincided with the onset of the symptoms suggestive of CMPA and the start of the elimination diet. The relative weight of children with CMPA continued to decrease compared with that in the non-allergic control group. In 2000, Agostoni et al. ( Reference Agostoni, Grandi and Scaglioni 23 ) compared the growth of 114 healthy infants with that of fifty-five infants with AD in which thirty-eight showed positive reactivity to milk proteins. Subjects affected by AD showed a progressive impairment of growth both in weight-for-age and length-for-age z scores. Differences between AD infants and healthy infants were significant from the second month of age onwards, more significantly in the second 6 months of life. More recently, Cho et al. ( Reference Cho, Hong and Lee 24 ) showed in 165 subjects with AD, of which seventy-seven were aged less than 12 months, that a higher number of sensitised food allergens was associated with negative effects on the growth and nutritional status of infants and young children with AD. Meyer et al. ( Reference Meyer, De Koker and Dziubak 25 ) assessed the growth status in ninety-seven food allergic children in the UK with a median (range) age of 27 (0·5–149) months. They found that elimination of more than three foods significantly affected the weight for age. Several other studies have shown growth deficit in infants with proved CMPA. Studies that have reported exact growth indices have been summarised in Table 8 ( Reference Agostoni, Fiocchi and Riva 3 , Reference Savino, Castagno and Monti 5 , Reference Isolauri, Sütas and Mäkinen-Kiljunen 13 , Reference de Boissieu and Dupont 26 – Reference Vandenplas, De Greef, Hauser and Paradice Study Group 28 ). The growth indices obtained in the present study are similar to the previously published data. Vieira et al. ( Reference Vieira, Morais and Spolidoro 29 ) reported the prevalence percentages of severe malnutrition in infants with CMPA aged less than 6 months: 16·5 % for weight-for-age (underweight), 27·8 % for length-for-age (stunting) and 13·9 % for weight-for-length z scores (wasting)( 30 ). In the present study, the prevalence of underweight (22·1 %) and stunted (31·7 %) children was higher, in contrast to wasted children (3·0 %), which was low in the CAMEL study.
Table 8 Summarised data relative to growth parameters in infants with cows' milk protein allergy (CMPA) at inclusion in the study (Mean values, standard deviations, number of subjects, percentages and 95 % confidence intervals)

AAF, amino acid-based formula; NR, not reported; SCORAD, scoring of atopic dermatitis; eHWF, extensively hydrolysed whey-based formula; CM, cows' milk; eHCF, extensively hydrolysed casein-based formula; RAST, radioallergosorbent test; SF, soya formula; eHRF, extensively hydrolysed rice-based formula; BF, breast-feeding; SBS, symptom-based score( Reference de Boissieu and Dupont 26 , Reference Vandenplas, De Greef and ALLAR study group 27 ).
* Days.
Recently, the National Institute for Health and Clinical Excellence guidelines( 31 ) for food allergy in children and young people were updated by new evidence concerning the impact of food allergies on growth in babies and infants. The Italian Society of Paediatric Nutrition published a position statement concerning the nutritional management and follow-up of infants and children with food allergy( Reference Giovannini, D'Auria and Caffarelli 32 ), showing an increased implication of scientific bodies in the assessment of physical growth in infants with CMPA.
Savino et al. ( Reference Savino, Castagno and Monti 5 ) assessed the nutritional adequacy of a rice-based hydrolysed formula, compared with infants fed a soya formula or an eHCF. The study evaluated the growth of fifty-eight infants with AD and CMPA (confirmed by an open challenge) who were fed either of these formulas during the first 2 years of life. The twenty-six infants fed the eHCF were included at a mean age of 3·33 (sd 2·31) months. Only weight-for-age z scores were reported. All z scores for infants fed the eHCF were higher than − 0·6. They increased between 2·5 and 5 months of age and from 7·5 to 24 months of age. In 2007, Agostoni et al. ( Reference Agostoni, Fiocchi and Riva 3 ) investigated in infants with CMPA whether the type of milk in the complementary feeding period (6–12 months of age) was associated with differences in the evolution of standardised growth indices (i.e. weight-for-age, length-for-age and weight-for-length z scores). For this, four feeding groups were compared, including one using a casein hydrolysate formula. Allergic infants (n 31), whose diagnosis was confirmed by a positive DBPCFC, were included between 5 and 6 months of age and fed an eHCF for 6 months. All z scores increased during this period of time: from − 0·44 to − 0·27 for the weight-for-age z score; from − 0·40 to − 0·16 for the length-for-age z score; from − 0·20 to − 0·12 for the weight-for-length z score. BMI-for-age z scores were not reported. Recently, thirty-four allergic infants, aged less than 6 months, fed an eHCF showed a significant improvement in their weight-for-age z score as of the first month of dietary treatment( Reference Vandenplas, De Greef and ALLAR study group 27 ). Altogether, these three studies and the present results, which are the largest reported to date, showed improvement of anthropometric data in allergic infants fed an eHCF. This demonstrates that these formulas are nutritionally adequate for allergic infants. According to paediatric guidelines, food allergic children with severe growth faltering should be fed amino acid-based formulas as first-line dietary treatment( Reference Koletzko, Niggemann and Arato 2 , Reference Fiocchi, Brozek and Schünemann 33 – Reference Muraro, Werfel and Hoffmann-Sommergruber 36 ). Results from the present study showed that the eHCF was well tolerated and enabled a growth catch-up in food allergic infants with poor growth at randomisation and favour guidelines recommending amino acid-based formulas mainly in case of intolerance to extensively hydrolysed formulas.
Questions have been raised as to whether the probiotic supplementation could have an effect on weight gain or not( Reference Million, Angelakis and Paul 37 ). Two recent meta-analyses conducted in healthy term infants have found that probiotics failed to significantly increase gains in weight, length and head circumference compared with the controls( Reference Mugambi, Musekiwa and Lombard 38 , Reference Szajewska and Chmielewska 39 ). Results presented here also showed that the probiotic supplementation had no effect on growth in infants allergic to CM, irrelevant of the nutritional status of the infant at study inclusion.
Conclusion
The randomised, double-blind, placebo-controlled study by the CAMEL study group included 119 infants allergic to CM. All the 119 infants clinically tolerated the formula well during the 4-week period preceding the follow-on study, including the 111 infants fed the eHCF for at least 7 months. In addition, the SCORAD index improved significantly during this period of time.
Standardised growth indices (z scores) were evaluated at randomisation and after 6 months of eHCF feeding. These results show that this eHCF is safe, hypo-allergenic (according to the standards of the American Academy of Paediatrics: tolerance by at least 90 % of CMPA infants with a 95 % CI) and nutritionally suitable for infants with CMPA.
Acknowledgements
The authors thank the infants, parents and physicians who kindly participated in the study.
The CAMEL study was funded by the Dutch Ministry of Economic Affairs. It had no role in the design and analysis of the present study or in the writing of this article.
The authors' contributions are as follows: C. D. participated in data analysis, drafting of the manuscript and final reviewing of the manuscript; J. H. and E. E. S. N. participated in the study design, data collection and final reviewing of the manuscript.
The authors declared that they have no conflicts of interest.
Appendix: CAMEL Study Group
Johan C. de Jongste, MD, PhD, Janneke N. Samsom, PhD, Eduard H. G. van Leer, MD, PhD, Beatrix E. E. Elink Schuurman, MScN, Lilian F. de Ruiter, Herman J. Neijens, MD, PhD.
Investigators in participating hospitals
F. G. A. Versteegh (Groene Hart Ziekenhuis, Gouda, The Netherlands), M. Groeneweg (Medisch Centrum Rijnmond Zuid, Rotterdam, The Netherlands), L. N. van Veen (Reinier de Graaf Groep, Delft, The Netherlands), A. A. Vaessen-Verberne (Amphia Ziekenhuis, Breda, The Netherlands), M. J. M. Smit (Juliana Kinderziekenhuis, Den Haag, The Netherlands), A. W. Vriesman and Y. M. Roosen (Albert Schweitzer Ziekenhuis, Dordrecht, The Netherlands) and G. L. den Exter (Vlietland, Schiedam, The Netherlands).
Healthy baby clinics
Consultatieburo Ouder & Kind (Rotterdam, The Netherlands), Vierstroom Zorgring (Gouda and Zoetermeer, The Netherlands), Maatzorg (Delft and Spijkenisse, The Netherlands), Thuiszorg Breda (Breda, The Netherlands), Stichting Opmaat (Zwijndrecht, The Netherlands), Thuiszorg Mark en Maasmond (Oosterhout, The Netherlands), Thuiszorg Nieuwe Waterweg Noord (Maassluis, The Netherlands), Thuiszorg De Zellingen (Capelle aan den IJssel, The Netherlands), and Thuiszorg West-Brabant (Roosendaal, The Netherlands).

















