Article contents
Novel one-pot synthesis of hierarchically porous Pd/C monoliths by a co-gelation method
Published online by Cambridge University Press: 05 March 2015
Abstract
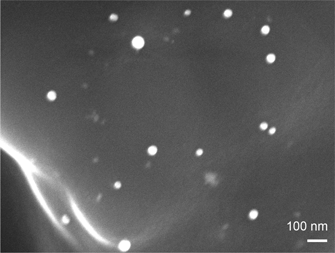
Described is a simple one-pot route for the synthesis of hierarchically porous carbon supported palladium (Pd/C) monoliths with a three-dimensional pore network via self-assembly under basic conditions of a resol polymer. Textural and morphological characterization of the Pd/C monoliths was carried out using nitrogen sorption analysis and scanning electron microscope. Formation of Pd nanoparticles ranging from 35 to 60 nm was observed. Evaluation of catalytic activity for styrene hydrogenation was performed using the Pd/C material as heterogeneous catalyst in batch mode. Studies revealed that the monoliths showed leaching and catalytic activity similar to a commercial Pd/C catalyst.
- Type
- Research Letters
- Information
- Copyright
- Copyright © Materials Research Society 2015
References
- 4
- Cited by




