Article contents
Effect of erosion speed on the interaction between erosion and corrosion of the Fe–3.5 wt% B alloy in a flowing zinc bath
Published online by Cambridge University Press: 17 March 2015
Abstract
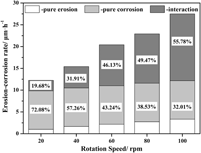
The effect of erosion speed on the interaction between erosion and corrosion of the Fe–3.5 wt% B alloy in a flowing zinc bath has been investigated using a rotating-disk technique. The total erosion–corrosion rate increases rapidly, whereas the pure erosion rate tends to increase linearly with an increase in erosion speed and with low damage. The increase in total erosion–corrosion rate is strongly dependent on erosion–corrosion interaction. During the erosion–corrosion process, the severe corrosion reaction roughens the surface by forming a loose corrosion layer and cracks in the anticaustic Fe2B skeleton, which eventually facilitates erosion. The micromechanical scouring effect of liquid zinc worsens corrosion by accelerating the removal of corrosion products and causing spallation of anticaustic Fe2B. An increase in erosion speed intensifies the micromechanical scouring effect of flowing zinc significantly. A strong erosion–corrosion interaction occurs at high erosion speed, which leads to a greater material loss rate.
- Type
- Articles
- Information
- Copyright
- Copyright © Materials Research Society 2015
References
REFERENCES
- 5
- Cited by




