To the Editor—Satisfactory quality control of sterilization processes is of paramount importance in maintaining the reliability of sterile supplies in a Central Sterile Supply Department (CSSD). The CSSD is a department in a healthcare facility that provides sterilized materials to wards, operating rooms, transplant units, and outpatient departments. The materials sterilized by a CSSD include dressing materials, surgical instruments, linens, endoscopes, and other equipment. The sterilizer used depends upon the nature of the item being sterilized, which may be heat resistant or heat sensitive.
The quality control process of each sterilizer varies depending upon the nature of the sterilizer. Herein, we describe our experience with the applications of various sterilization indicators in a CSSD in a 167-bed oncology hospital in eastern India. 1 , Reference Basu, Bhattacharya, Mahajan, Ramanan and Chandy 2 Our discussion includes cost implications and quality control issues. Physical parameters and chemical and biological indicators are monitored in this center according to the recommendations of the Association for the Advancement of Medical Instrumentation (AAMI) and the International Standards Organization (ISO). 3 , 4
The physical monitoring system depends upon sterilization time, temperature, and pressure. In daily work practice, this monitoring can be performed by visual inspection of the gauge-glass in an analog meter, via a program-linked control (PLC system), or with an automatic printout mechanism. The system is calibrated by inserting thermocouples inside the chamber or using a data logger with the manufacturer’s predetermined specifications. The data printouts from each sterilizer are documented in the register along with the data for other items sterilized in the same load.
The chemical monitoring system is composed of a set of various indicators based on specific requirements such as equipment monitoring (Bowie-Dick test pack, Class II CI), pack monitoring (internal chemical indicators, Class III-VI), and exposure monitoring (exposure control tape, Class I CI), and others. Every chemical indicator (CI) has 1 stated end-point value at which a color change occurs. However, Class V CIs must have 3 stated values: at 121°C, at 135°C, and at 1 temperature between these values (at which killing of the biological indicator is achieved). Class VI CIs only have 1 stated value for cycle-specific sterilization, depending on the plateau time. All of the chemical indicators are tested using a chemical indicator evaluating resistometer, and each manufacturer follows the ISO 11140-1 standard. 3 , 4 A good-quality chemical indicator can easily detect steam quality, non-condensable gases, and proper sterilant penetration inside the sterilizer. Currently, some chemical indicators are also used to check hollow load devices for proper sterilant penetration in a vacuum sterilizer. The air trapped inside the lumen of a hollow device is calculated as lumen diameter multiplied by lumen length, which is called hollow penetration resistance. The shelf life of unused chemical indicators varies with different manufacturers and depends on ambient temperature and humidity level.
The biological monitoring system depends on live nonpathogenic bacterial spores and stringent sterilization requirements. The biological indicators are prepared using a live bacterial spore strip containing a minimum of 1 million live spores. All of the biological indicators are approved by the American Type Culture Collection and are also tested using the biological indicator evaluator resistometer. The D-value of a biological indicator is defined as “the time required in minutes at a certain temperature (121°C) to reduce the number of viable microorganisms by a factor of 10,”Reference Kamolsiripichaiporn, Subharat, Udon, Thongtha and Nuanualsuwan 5 and the Z-value of a biological indicator is defined as “the number of degrees in Centigrade to reduce the D-value with a factor of 10,”Reference Kamolsiripichaiporn, Subharat, Udon, Thongtha and Nuanualsuwan 5 whereas the sterilization assurance level is the “probability of a single unit being non-sterile after it has been subjected to sterilization.” 3 , 4 Finally, the entire set of biological indicators after sterilization is sent to the microbiology department for sterility assurance testing.
With regard to consumables expenditures, the cost implications for the physical monitoring system is negligible and consists of the cost of the thermal paper for printouts. Calibration for physical monitoring should be done each year by an approved external agency.
The cost of chemical indicators is manufacturer based (eg, 3M, USA; Johnson & Johnson, USA; Gke, Germany) (Table 1). This cost depends on how many critical variables are being measured by the indicators. In our facility, no failures of chemical or biological indicators were recorded in 2013. The total cost of biological indicators was Rs 194,054 (US$3,234; 16% of the total indicator expense); this cost is minor compared to the cost of chemical indicators, which was Rs 10,31,616 (US$17,194; 84% of total indicator expense). In our center, these costs are supported by the institution as part of the quality-related expenditures of the CSSD. The chemical indicators are used rationally based on the type of materials to be sterilized. For surgical instruments, we use Class V and Class VI indicators, whereas for items such as dressing sets, Class III and Class IV indicators are used. For dressing materials and linens, we use Class I indicators. These classifications are based on Spaulding’s classification of criticality of materials with regard to infection control and cost considerations. In our CSSD, the total indicator cost was estimated to account for 6.3% of the total annual operating cost of the CSSD, and this was approximately 34% of the total annual consumable cost. These figures have significant implications for CSSDs and hospital administrators. Ensuring quality in sterilization processes requires resources of considerable magnitude. The challenge is to ensure that the sterilization indicators are used efficiently to prevent wastage and maintain quality.
Table 1 Sterilization Indicators in Central Sterile Supply Department at Tata Medical Center
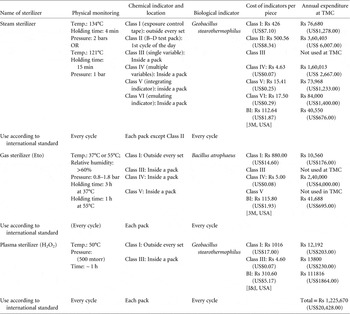
Note. TMC, Tata Medical Center; Rs, rupees; BI, biological indicator; J&J, Johnson & Johnson. Class I: temperature; Class II: temperature, pressure, time, steam quality, non-condensable gas; Class III: temperature; Class IV: temperature and time; Class V: time, steam, temperature; Class VI: time, steam, temperature (cycle specific).
ACKNOWLEDGMENTS
Financial support. None reported.
Conflict of interest. None reported.





