Neurobiological mechanisms of bipolar disorder (BD) are still unclear and subject to debate. One way to decipher these mechanisms is the use of neuroimaging. Magnetic resonance imaging (MRI) and positron emission tomography (PET) both allow the study of structural and functional alterations in BD. Structural neuroimaging techniques include anatomical T1 and diffusion weighted MRI. Studies of BD patients with T1 MRI revealed alterations in volume of prefrontal and limbic regions such as the amygdala, the parahippocampal and the cingulate cortices (Houenou et al. Reference Houenou, D'Albis, Vederine, Henry, Leboyer and Wessa2012). Diffusion MRI studies identified in BD microstructural white matter abnormalities in prefrontal–limbic (uncinate), limbic (cingulum) and also callosal tracts (Sarrazin et al. Reference Sarrazin, Poupon, Linke, Wessa, Phillips, Delavest, Versace, Almeida, Guevara, Duclap, Duchesnay, Mangin, Le Dudal, Daban, Hamdani, D'Albis, Leboyer and Houenou2014). Most of the functional neuroimaging studies used emotion processing paradigms during functional MRI (fMRI) and generally reported hyperactivity of the subcortical limbic structures associated with a hypoactivation of prefrontal cortices, even in euthymic patients (Delvecchio et al. Reference Delvecchio, Sugranyes and Frangou2013). These results have led to the conceptualisation of neural models of BD (Table 1): in response to emotional stimuli, these models assume a hyperactivity of the subcortical limbic structures implicated in automatic emotional processing (such as the amygdala and the parahippocampal cortex). Owing to altered structural and functional connectivity between ventral prefrontal networks and these limbic regions, the modulation of the activity of the amygdala by the prefrontal cortices would be inefficient. This altered prefrontal control over an overactive limbic system may trigger emotional hyper-reactivity and mood episodes (Phillips & Swartz, Reference Phillips and Swartz2014).
Table 1. Some selected key neuroimaging studies and models of BD
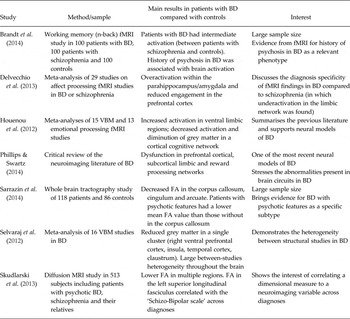
BD, bipolar disorder; FA, fractional anisotropy; VBM, voxel-based morphometry.
Despite these promising results and neural models of BD, several caveats remain. The aetiology of the identified abnormalities (genetic or environmental; neurodevelopmental or neurodegenerative) is one of these. Their specificity (as compared to unipolar depression or schizophrenia) is a second one. Another unsolved issue is the heterogeneity of the results between studies. Recent meta-analytic analyses identified this heterogeneity as large (Selvaraj et al. Reference Selvaraj, Arnone, Job, Stanfield, Farrow, Nugent, Scherk, Gruber, Chen, Sachdev, Dickstein, Malhi, Ha, Ha, Phillips and Mcintosh2012). Three types of factors can explain it: first, the small samples used lead to the inflation of type I error (false positives) and low reproducibility of the results (Button et al. Reference Button, Ioannidis, Mokrysz, Nosek, Flint, Robinson and Munafo2013). Second, methodological factors such as different MRI scanners, field strengths, acquisition sequences or processing software can lead to different results between studies. Third, clinical differences between the samples may account for a significant proportion of this heterogeneity. Potential different subtypes of BD can arise from various clinical features: differences in age at onset, presence/absence of suicide attempts, BD type I or II, presence/absence of psychotic symptoms during episode, variability in emotional reactivity, medication effects (lithium is supposed to have a neuroprotective effect), comorbidities (especially substance use and anxiety disorders), presence/absence of family history of mood disorders or presence/absence of environmental factors, such as childhood trauma, urbanicity during upbringing, migration. The potential sources of clinical heterogeneity are thus numerous and diverse.
We suggest two different strategies to understand the link between these clinical factors and brain alterations. First, the recruitment of very large samples will allow the study of this link in BD. To this regard, a few recent studies have used sample sizes N > 100 which had the statistical power to explore the impact of psychotic symptoms (Brandt et al. Reference Brandt, Eichele, Melle, Sundet, Server, Agartz, Hugdahl, Jensen and Andreassen2014; Sarrazin et al. Reference Sarrazin, Poupon, Linke, Wessa, Phillips, Delavest, Versace, Almeida, Guevara, Duclap, Duchesnay, Mangin, Le Dudal, Daban, Hamdani, D'Albis, Leboyer and Houenou2014). Their results support that BD with psychotic features may be a subtype of BD with specific neurobiological basis, evidenced by poorer white matter integrity. Second, we suggest the use of innovative clinical approaches for phenotyping samples in neuroimaging studies. Among these, one of the most promising is the dimensional clinical assessment of BD and related disorders. Its aim is to identify biologically based clinical dimensions that will be more relevant to the study of BD and should be independent of diagnostic categories. The National Institute of Mental Health has recently advocated for this strategy, with its Research Domain Criteria initiative. A very recent study found that fractional anisotropy (a measure of the integrity of white matter tracts) in the left superior longitudinal fasciculus correlated with the ‘Schizo-Bipolar scale’ across diagnoses of schizophrenia or BD with psychotic features. This scale is a measure of the dimensional continuum between schizophrenia and BD (Skudlarski et al. Reference Skudlarski, Schretlen, Thaker, Stevens, Keshavan, Sweeney, Tamminga, Clementz, O'Neil and Pearlson2013). This shows the feasibility of correlating a dimensional measure to a neuroimaging variable across diagnoses. Another example is executive dysfunction: Shpeherd et al. recently found that patients (with BD or schizophrenia) with executive deficits had grey matter reduction in frontal and precentral gyri compared to patients without executive dysfunction (Shepherd et al. Reference Shepherd, Quide, Laurens, O'Reilly, Rowland, Mitchell, Carr and Green2014).
The choice of the pertinent clinical dimensions to correlate with MRI data is determinant. Despite increasing sample sizes, neuroimaging studies will not be able to test many dimensions, due to multiple comparison issues and feasibility reasons. Epidemiological and clinical studies will thus have a critical role in suggesting what may be the ‘core’ clinical dimensions to study. One example is the recent identification of emotional hyper-reactivity as a candidate of such a biologically based core dimension of mood disorders (Phillips & Kupfer, Reference Phillips and Kupfer2013). Several lines of evidence point towards a critical role of emotional reactivity in BD and mood switches. Emotional reactivity is increased in patients with BD across thymic states, but also in other disorders such as borderline personality. Studies on neuroticism (that can be conceptualised as emotional reactivity to negative stimuli) suggest that emotional reactivity to negative stimuli is associated with neuroanatomical and functional characteristics in the limbic network. It remains to be tested if emotional reactivity is a clinical dimension associated with changes in the limbic system across diagnoses. Another example of this approach is the recent identification of neural correlates of the epidemiological risk factors for schizophrenia such as urban upbringing, migration, cannabis use or childhood trauma (Lederbogen et al. Reference Lederbogen, Kirsch, Haddad, Streit, Tost, Schuch, Wust, Pruessner, Rietschel, Deuschle and Meyer-Lindenberg2011). Although most of these risk factors are shared with BD, we still do not know if these neural correlates are valid across diagnoses.
In conclusion, neuroimaging studies have allowed us to build models of the neural mechanisms of BD. A deeper understanding of how risk factors and clinical dimensions impact the brain and lead to mental disorders such as BD necessitate a strong collaboration between epidemiologists, clinicians and neuroscientists.
Acknowledgements
None.
Financial Support
J.H. was partially supported by the Investissements d'Avenir programs managed by the ANR under references ANR-11-IDEX-0004- and ANR-10-COHO-10-01 P.B. was partially supported by grants from the Italian Ministry of Health (grant no. GR-2010-2319022; GR-2010-2316745; RF-2011-02352308).
Conflict of Interest
None.
Ethical Standards
The authors declare that no human or animal experimentation was conducted for this work.





