Article contents
Designing soft nanomaterials via the self assembly of functionalized icosahedral viral capsid nanoparticles
Published online by Cambridge University Press: 02 December 2014
Abstract
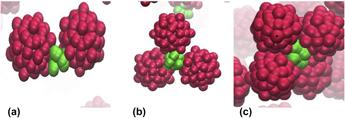
Through implicit solvent coarse-grained molecular dynamics simulations, we investigate the equilibrium morphologies resulting from the self-assembly of building blocks composed of anisotropically functionalized icosahedral viral capsid nanoparticles (NPs). We investigate the self-assembled aggregate morphologies for variations in the functional group chain length and solvent quality. We observe specific building block architectures to favor the formation of n-mers, chain- and network-like structures. Our work is in agreement with the earlier simulation studies on icosahedral gold nanocrystals that generate self-assembled chain-like structures. [G. Bilalbegovic, Comput. Mater. Sci.31, 181 (2004).] In addition, our results agree with those by Finn et al., who have shown small predominantly chain-like aggregates with mannose-decorated cowpea mosaic virus (CPMV) [K.S. Raja, Q. Wang, and M.G. Finn, ChemBioChem4, 1348–1351 (2003)] and small aggregates with oligonucleotide functionalization on the CPMV capsid. [E. Strable, J.E. Johnson, and M.G. Finn, Nano Lett.4, 1385–1389 (2004).] Visual inspection suggests that our results most likely span the low temperature limits explored by Finn et al. and show a good degree of agreement with the experimental results at an annealing temperature of 4 °C. [E. Strable, J.E. Johnson, and M.G. Finn, Nano Lett.4, 1385–1389 (2004).] Our investigations reveal the possibility of novel n-mer type aggregates that could be synthesized using icosahedral NPs with appropriate surface functionalization and solvent conditions.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 30 , Issue 1: Focus Issue: Soft Nanomaterials , 14 January 2015 , pp. 141 - 150
- Copyright
- Copyright © Materials Research Society 2015
References
REFERENCES
- 5
- Cited by




