Article contents
Asynchronous stoichiometric response in lithium iron phosphate batteries
Published online by Cambridge University Press: 11 November 2014
Abstract
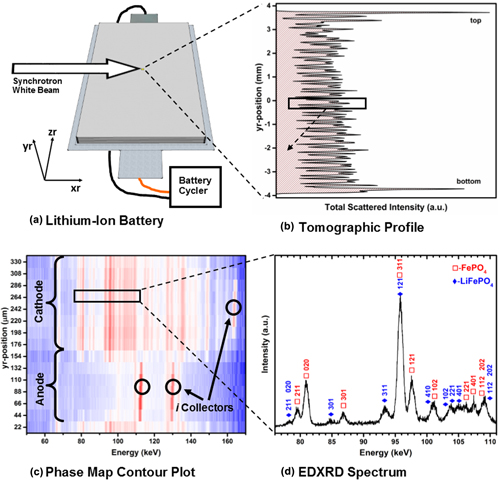
Operando energy-dispersive x-ray diffraction (EDXRD) was carried out on a newly formed 8 Ah lithium iron phosphate (LiFePO4) battery with the goal of elucidating the origin of asynchronous phase transformation commonly seen with in situ x-ray diffraction studies. The high-energy photons at the NSLS X17B1 beamline allow for penetration into a fully assembled battery and therefore negate any need for a specially designed in situ cell which often uses modified current collectors to minimize x-ray attenuation. Spatially-and-temporally resolved phase-mapping was conducted with a semiquantitative reference intensity ratio (RIR) analysis to estimate the relative abundance of the delithiated phase. The data show an asynchronous response in the stoichiometry versus the electrochemical profile and suggest limited diffusion in the electrode toward the end of discharge. Our results confirm that the asynchronous electrode response is not just limited to specially designed cells but occurs in fully assembled cells alike. We attribute this behavior to be a consequence of performing a local measurement over a wide-area heterogeneous reaction.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 30 , Issue 3: Focus Issue: In-situ and Operando Characterization of Materials , 14 February 2015 , pp. 417 - 423
- Copyright
- Copyright © Materials Research Society 2014
References
REFERENCES
- 8
- Cited by




