Article contents
Fluorescent π-conjugated polymer nanoparticles: A new synthetic approach based on nanoagglomeration via polyion association
Published online by Cambridge University Press: 23 September 2014
Abstract
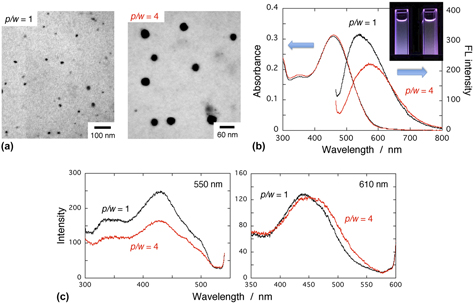
A facile method is developed to synthesize ion-based π-conjugated polymer nanoparticles. The polymer chosen is anionic poly(2-methoxy-5-propyloxysulfonate phenylene vinylene); MPS-PPV. The synthesis is based on nanoagglomeration via polyion association (termed as NAPA method) that includes polyion complex formation and subsequent globulization to fabricate nanoarchitectures in water-based liquid. Salient features include no spectral shift in the UV–vis absorption upon the nanoparticle formation, in contrast to a common observation that conjugated polymer nanoparticles made by a reprecipitation method exhibit blue shift in their peak. This behavior suggests the absence of further bending or kinking of the polymer backbone during nanoglobulization. Fluorescence peak energy is size dependent; the larger the particle size is, the lower is its fluorescence energy. This can be dominantly ascribed to the increased contribution of sole excitation of the chromophoric segments that have a long effective conjugation length. Because a small (large) particle has a large (small) surface-to-volume ratio, the blue-site (red-site) fluorescence is associated with the surface (inner) region of the polymer nanoparticles, respectively.
- Type
- Articles
- Information
- Journal of Materials Research , Volume 30 , Issue 1: Focus Issue: Soft Nanomaterials , 14 January 2015 , pp. 10 - 18
- Copyright
- Copyright © Materials Research Society 2015
References
REFERENCES
- 2
- Cited by




