Gestational diabetes mellitus (GDM), which occurs in approximately 1–5 % of pregnancies in China( Reference Wei, Yang and Gao 1 ), is defined as any degree of glucose intolerance that emerges at the onset of pregnancy or is first recognized during pregnancy( Reference Buchanan and Xiang 2 ). Pregnant women diagnosed with GDM early in their pregnancies are at a higher risk of obstetric complications such as macrosomia, hydramnios, ketoacidosis, hypertension, preterm delivery and caesarean delivery( Reference Yang, Hsu-Hage and Zhang 3 ). Moreover, GDM increases the risk of subsequent type 2 diabetes for the mother and child later in life( Reference Yang, Hsu-Hage and Zhang 3 ). Due to a rapid increase in GDM incidence in China( Reference Zhang, Dong and Zhang 4 ), both prevention of the development of GDM and glycaemic control in GDM patients are urgently needed( Reference Wei, Yang and Gao 1 , Reference Case, Willoughby and Haley-Zitlin 5 ).
Dietary intervention is considered the preferred treatment to achieve normal glucose levels and to control excessive fetal growth and other associated adverse outcomes in GDM patients( Reference Crowther, Hiller and Moss 6 , Reference Evans and Patry 7 ). General dietary treatment measures for GDM patients are largely focused on controlling total energy intake and promoting consumption of foods with low fat content (particularly low saturated fat) and low glycaemic index (GI), with moderate restriction of carbohydrate( Reference Louie, Brand-Miller and Moses 8 ). One randomized controlled trial (RCT) has shown that a low-GI diet significantly reduced the need for the use of insulin in sixty-three GDM women( Reference Gunderson 9 ). Another RCT showed that a low-GI diet was more efficient in the control of postprandial glucose than the high-GI diet in forty-seven women with gestational hyperglycaemia( Reference Grant, Wolever and O’Connor 10 ). Similar results were observed in patients with general type 2 diabetes mellitus( Reference Monro 11 ). The results of a systematic review of six RCT suggested that a low-GI diet more significantly decreased body mass, total fat, total cholesterol (TC) and LDL cholesterol (LDL-C) than other dietary programmes in overweight or obesity( Reference Thomas, Elliott and Baur 12 ). Another review also showed that a low-GI diet efficiently improved glycaemic control in diabetes without causing hypoglycaemic events( Reference Thomas and Elliott 13 ). These findings suggest that the low-GI diet might be a useful measure for glycaemic control and the improvement of other cardiovascular risk factors in GDM women.
However, there exist different results in related studies. One RCT in 107 GDM patients showed that a low-GI diet (compared with the intake of all types of carbohydrates with varying GI) was not more efficient in the improvement of glycaemic control( Reference Perichart-Perera, Balas-Nakash and Rodríguez-Cano 14 ). Inconsistent results were observed in other studies( Reference Moreno-Castilla, Hernandez and Bergua 15 ), possibly due to insufficient appreciation of the total amount of carbohydrate in the foods, which is the major determinant for blood glucose response.
Dietary glycaemic load (GL) was introduced to quantify the total glucose-increasing potential of carbohydrate-containing foods( Reference Salmeron, Manson and Stampfer 16 ). GL is calculated as the product of GI multiplied by the available carbohydrate proportion of the food( Reference Lajous, Boutron-Ruault and Fabre 17 ). Accumulating evidence has indicated that low-GL diets based on general dietary treatments are more promising educational intervention strategies in patients with diabetes because both GI and total consumption of carbohydrate are considered( Reference Miller, Edwards and Kissling 18 ). However, limited evidence is available from GDM patients, especially among Asian or Chinese populations. A pilot RCT (n 46) demonstrated that a low-GL diet improved maternal cardiovascular risk factors compared with a low-fat diet( Reference Rhodes, Pawlak and Takoudes 19 ), which implies that a low-GL diet might be favourable to the outcomes of GDM. Further studies with larger sample size are warranted to evaluate whether a low-GL diet intervention during pregnancy would be more effective than other dietary intervention programmes.
The present study aimed to compare the effects of a low-GL dietary intervention and a general dietary intervention on glycaemic control and lipid metabolism in GDM women and on maternal and neonatal outcomes.
Experimental methods
Study population
In total, ninety-five participants were recruited for the current RCT by screening 2540 outpatients at the Center of Maternal Primary Care in Guangdong General Hospital, China, from June 2008 to July 2009. Eligible participants were required to be a resident of Guangzhou, the provincial capital of Guangdong; aged between 18 and 40 years; and an incident GDM patient diagnosed at 24–26 weeks of gestation. GDM patients were screened with a 50 g glucose challenge test according to the guidelines of the Chinese Medical Association( 20 ) and the American Diabetes Association( 21 , 22 ). Positive cases (glucose concentration ≥7·8 mmol/l following the glucose challenge test) were confirmed by further evaluation with a 3 h, 75 g oral glucose tolerance test and were diagnosed as GDM patients when they met at least two of the following criteria for glucose concentration at 0, 1, 2 and 3 h post-load: fasting, >5·8 mmol/l; 1 h, >10·6 mmol/l; 2 h, >9·2 mmol/l; and 3 h, >8·1 mmol/l. The exclusion criteria included: (i) pre-pregnancy diabetes; (ii) multiple gestations; (iii) other severe diseases, including hypertension, chronic hepatic and kidney disease and cancer; (iv) use of insulin or hypoglycaemic medications; (v) less than 9 years of formal schooling; and (vi) previous intensive nutrition education or intervention for diabetes. The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects/patients were approved by the Human Research Ethics Committee of Guangdong General Hospital. Written informed consent was obtained from all subjects/patients.
Randomization and interventions
Eligible participants were randomly assigned to one of the two arms according to odd/even random numbers generated by Excel® software: the Control group and the low-glycaemic-load intervention (Low-GL) group. Both of these groups received a one-on-one general dietary intervention every two weeks according to the guidelines recommended by the Chinese Medical Association( 20 ) from 24–26 gestational weeks until delivery, which was usually 12–14 weeks later. The general dietary intervention was made via detailed advice and the provision of sample daily menus that mainly targeted limitations on starches and fat and encouraged appropriate macronutrient proportion ranges. The recommended daily energy intake was approximately 146 kJ (35 kcal)/kg per d for individuals with a normal weight and 104 kJ (25 kcal)/kg per d for obese women (BMI≥28 kg/m2) according to their pre-pregnancy weight( 20 , Reference Bei-Fan 23 ). The percentages of energy from carbohydrate, protein and fat were controlled to 45–50 %, 20–24 % and 25–30 %, respectively. Five to six meals daily with smaller portions were also recommended.
In addition to general dietary advice, the participants enrolled in our study also received instruction on the glycaemic effects of food. These individuals were given an exchange list from which they selected their starch choice or serving (Control or Low-GL). The exchange lists were designed based on the key foods strategy. The lists provided to the control group comprised intermediate- to high-GL foods, which represent the typical Guangzhou diet, whereas the lists provided to the Low-GL group contained low-GL foods. Because milk products, vegetables and fruits are recommended for pregnant women to maintain fetal growth and development, participants were also given advice on the GL of starchy fruits and vegetables high in starch. The GL value of each food was obtained from an internationally published GL table( Reference Foster-Powell, Holt and Brand-Miller 24 ) and the food composition table of China (2002)( Reference Yang, Wang and Pan 25 ). The average GI/GL values for each participant were calculated( Reference van Woudenbergh, Kuijsten and Sijbrands 26 ) as follows:
 $$\eqalignno{{\rm Mean \ GI}=\mathop{\sum} & {\rm (food}\;{\rm GI}{\times}{\rm amount}\,{\rm of}\,{\rm carbohydrate } \cr & {\rm contained}\,{\rm in}\,{\rm a}\,{\rm specified}\,{\rm food)/total } \cr & {\rm amount}\,{\rm of}\,{\rm carbohydrate} $$
$$\eqalignno{{\rm Mean \ GI}=\mathop{\sum} & {\rm (food}\;{\rm GI}{\times}{\rm amount}\,{\rm of}\,{\rm carbohydrate } \cr & {\rm contained}\,{\rm in}\,{\rm a}\,{\rm specified}\,{\rm food)/total } \cr & {\rm amount}\,{\rm of}\,{\rm carbohydrate} $$
and
Each participant received one copy of Dietary Guidance Handbook for GDM Women, which compiles specific advice. The handbooks for the Control and Low-GL groups had the same cover, format and length but contained different exchange lists on food GL. Dietitians assessed dietary intakes using a 3 d recall to assess the compliance once every two weeks and reinforced the intervention at each visit. The exact content of the intervention was altered to meet individual needs, based on dietary details and weight growth between the two interventions.
All participants were asked not to consume alcohol or dietary supplements or medications that could influence glucose tolerance and lipid metabolism and were told to maintain their usual exercise patterns during the study.
Dietary intake assessments
Habitual dietary intake pre-treatment and during the interventional period was assessed with an FFQ using the reference times of the past year at baseline and of the intervention period at the end of the intervention( Reference Zhang and Ho 27 ). Dietary intake was calculated according to the 2002 China food composition table( Reference Yang, Wang and Pan 25 ).
Outcome measurements
The primary outcomes were fasting plasma glucose (FPG; mmol/l) and glycated Hb (HbA1c; %) and 2 h postprandial blood glucose (2 h PG; mmol/l). Secondary outcomes included fasting serum lipid levels, body weight, BMI and gestational outcomes (such as preterm delivery, macrosomia, intra-uterine asphyxia, eclampsia, postpartum haemorrhage and perinatal infection).
Venous or postprandial blood samples were collected before and after the intervention at admission to the hospital for delivery. Serum was separated by a centrifugation procedure (3000 rpm for 10 min) after clotting at room temperature. The samples were processed and analysed by the Central Laboratory of Guangdong General Hospital.
Glucose levels were determined by the oxidase method within 2 h after sampling. Glucose, TC, TAG, HDL cholesterol (HDL-C) and LDL-C were assessed using an LX-20 automatic biochemical analyser (Beckman Coulter Trifel). HbA1c levels were assessed by HPLC. The inter-assay CV ranged between 2·0 % and 5·0 %.
Pre-pregnancy weight was self-reported on the first day of prenatal care. Body weight and height were measured with the participants wearing only undergarments.
Statistical analysis
The statistical software package SPSS 13·0 was used to analyse the data. The data are reported as mean and standard deviation, or as number and percentage. The t test or χ 2 test or Fisher’s exact test were used for the comparisons of baseline characteristics. Differences in mean changes (follow-up value minus baseline value) in the outcomes over the intervention period and differences in other continuous data between the two arms were examined using a t test for normally distributed data or Wilcoxon’s rank test for non-normally distributed data. A modified intention-to-treat principle including all participants who completed the baseline and follow-up assessments was used in the analysis of the primary outcomes as done in a previous report( Reference Kreijkamp-Kaspers, Kok and Grobbee 28 ). All P values were two-sided. The level of significance was set at P<0·05.
Results
Demographic characteristic of the participants
Of the ninety-five GDM participants, eighty-three completed the entire intervention and data collection processes, whereas the other twelve dropped out and did not complete post-intervention tests for the following reasons: six could not comply with the dietary schedule, three needed insulin treatment due to blood glucose that they could not control properly, one experienced pre-eclampsia, one experienced severe gestational hypertension and one refused to continue the study for an unexplained reason. There were no differences in demographic characteristics or pre-intervention metabolic measurements between completers and dropouts. The study flow chart is shown in Fig. 1.
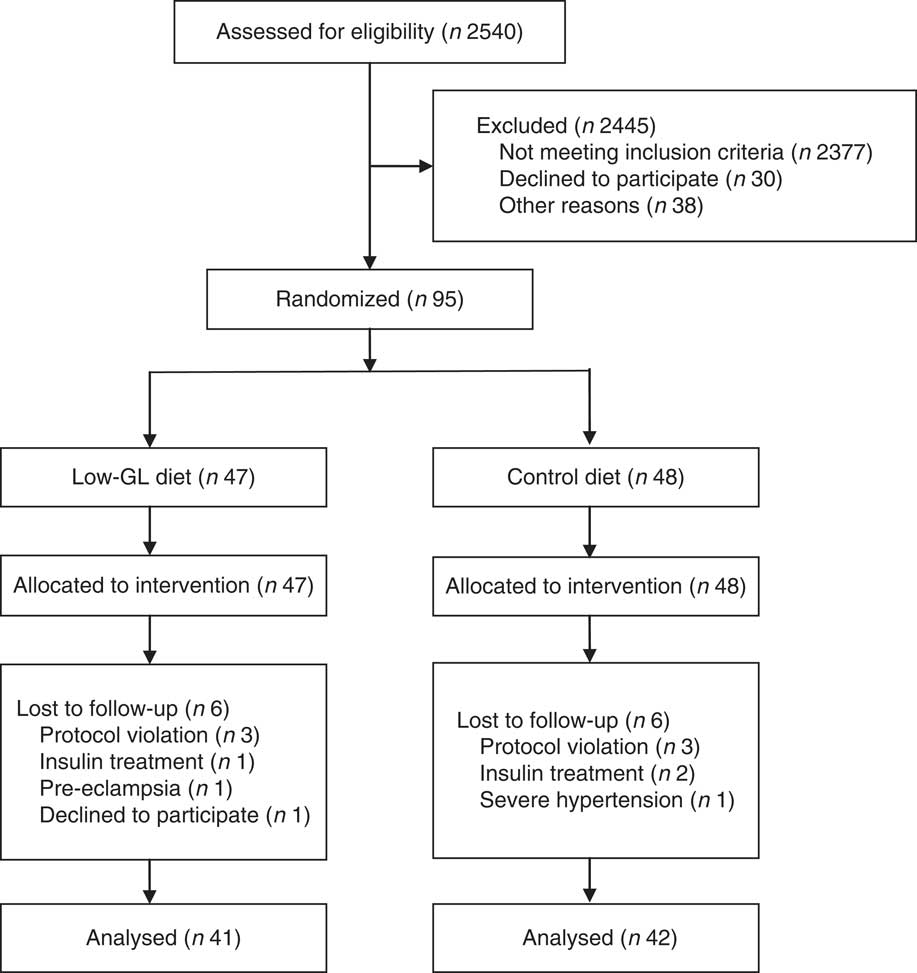
Fig. 1 The study flow diagram
The demographic characteristics of the sample are shown in Table 1. The majority of participants (89 %) had a college degree or higher. Most participants (95 %) had no adverse reproductive history and 76 % had no family history of diabetes. There were no significant differences in age, pre-pregnancy BMI, educational attainment, family history of diabetes, gestational week at diagnosis and at enrolment in the intervention, and adverse reproductive history between the two groups at baseline (all P>0·05).
Table 1 Baseline characteristics of the study participants: women with gestational diabetes mellitus recruited at the Center of Maternal Primary Care in Guangdong General Hospital, China, from June 2008 to July 2009

GL, glycaemic load.
* The χ 2 test was used for the group comparisons. For the others, the t test was used.
Daily intakes of energy and macronutrients before and after the intervention
No significant differences were observed between the two groups in the dietary intakes of energy, protein, fat or carbohydrate, or in dietary fibre, or in dietary GI or GL at baseline (Table 2). After the intervention, the intake of energy of all participants dropped by approximately 21 %. The greatest reduction occurred in carbohydrate intake (approximately 28 %, P<0·01), followed by the intake of fat (approximately 15 %, P<0·01) and protein (approximately 10 %, P<0·01). GI and GL in both groups decreased as well. GI decreased by 2·3 (sd0·2) in the Control group and by 5·9 (sd0·2) in the Low-GL group (P<0·01), and GL decreased by 62·6 (sd5·2) in the Control group and by 67·4 (sd6·2) in the Low-GL group (P<0·01). After the intervention, the Low-GL group had significantly lower values of GL (122 v. 136) and GI (50 v. 54) but greater dietary fibre (33 v. 29 g/d) than did the Control group (all P<0·01). No significant differences were observed in the dietary intakes of energy, protein, fat or carbohydrate between the two groups (Table 2). There was no significant difference in the intakes of micronutrients between the two groups before and after the intervention (data not shown).
Table 2 Intakes of energy and macronutrients and the values of GI and GL before and after the dietary intervention according to study group: women with gestational diabetes mellitus recruited at the Center of Maternal Primary Care in Guangdong General Hospital, China, from June 2008 to July 2009
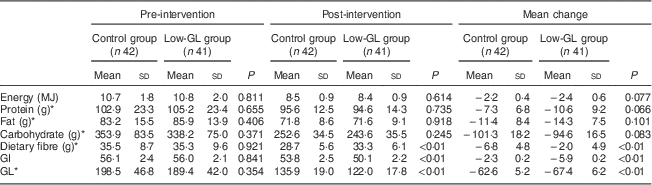
GI, glycaemic index; GL, glycaemic load.
The Control group received an individualized general dietary intervention and the Low-GL group received an intensive low-GL intervention, every two weeks, from 24–26 weeks of gestation to delivery.
* Wilcoxon’s rank test was used for the group comparisons. For the others, the t test was used.
Comparison of mean changes in blood metabolic outcomes
As shown in Table 3, there were no significant differences in the metabolic outcomes at baseline (all P>0·05). After the intervention, significantly greater decreases in FPG (−0·33 v. −0·02 mmol/l, P<0·01) and 2 h PG (−2·98 v. −2·51 mmol/l, P<0·01) were observed in the Low-GL group compared with the Control group. Significantly lower increases in TC (0·12 v. 0·23 mmol/l) and TAG (0·41 v. 0·56 mmol/l) and a significantly lower decrease in HDL-C (−0·01 v. −0·11 mmol/l) were also observed in the Low-GL group compared with the Control group (all P<0·05). Our study had a power of over 0·85 to detect the above-mentioned differences at a significance level of 0·05. However, no significant difference was observed in the mean changes of HbA1c and LDL-C between the two groups over the intervention period (P>0·05).
Table 3 Metabolic outcomes before and after the dietary intervention according to study group: women with gestational diabetes mellitus recruited at the Center of Maternal Primary Care in Guangdong General Hospital, China, from June 2008 to July 2009

GL, glycaemic load; FPG, fasting plasma glucose; 2 h PG, 2 h postprandial glucose; HbA1c, glycated Hb; TC, total cholesterol; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol.
The Control group received an individualized general dietary intervention and the Low-GL group received an intensive low-GL intervention, every two weeks, from 24–26 weeks of gestation to delivery.
* Wilcoxon’s rank test was used for the group comparisons. For the others, the t test was used.
Maternal–fetal outcomes
Total weight gain and its average value per week, as well as the birth weight of infants were similar in the two groups (all P>0·50). There was no significant difference in the incidence of maternal–fetal perinatal outcomes, including preterm delivery, macrosomia, intra-uterine asphyxia, postpartum haemorrhage and infection, between the two arms (all P>0·70; Table 4).
Table 4 Maternal–fetal perinatal outcomes after the dietary intervention according to study group: women with gestational diabetes mellitus recruited at the Center of Maternal Primary Care in Guangdong General Hospital, China, from June 2008 to July 2009

GL, glycaemic load.
The Control group received an individualized general dietary intervention and the Low-GL group received an intensive low-GL intervention, every two weeks, from 24–26 weeks of gestation to delivery.
* Fisher’s exact test was used for the group comparisons. For the others, the t test was used.
Discussion
The present RCT found that a low-GL (with higher dietary fibre) intervention significantly improved FPG, 2 h PG and fasting TC, TAG and HDL-C compared with a general dietary intervention in GDM. Our findings suggest that a low-GL targeted dietary intervention is more effective for improving glycaemic control and lipid levels in GDM women.
Based on these study observations, the improvements in metabolic outcomes of the Low-GL group were promising. The study found significantly larger reductions in FPG and 2 h PG in GDM women who were prescribed a low-GL diet compared with a high-GL diet, and had an isoenergetic intake and the same carbohydrate intake. A previous study that explored blood glucose control by a low-GI diet in women with gestational hyperglycaemia also demonstrated that more participants achieved their postprandial glucose target on a low-GI diet (58·4 %) than on a control diet (48·7 %, P<0·001)( Reference Grant, Wolever and O’Connor 10 ). The advantage for diabetes control in the present study was observed despite a lack of significant differences in HbA1c between the two groups.
HbA1c is the ‘good criterion’ for measuring the effect of diabetes treatment( Reference Goldstein, Little and Lorenz 29 ). Based on our study’s observations, however, we found that HbA1c increased, but not significantly, in these two groups after a 10- to 12-week diet intervention compared with baseline. Similarly, a 12-month randomized controlled comparison of low-GI, high-GI and low-carbohydrate diets in patients with type 2 diabetes found no differences between the groups in HbA1c( Reference Wolever, Gibbs and Mehling 30 ). Christensen et al.( Reference Christensen, Viggers and Hasselström 31 ) performed medical nutrition therapy with or without fruit restriction in type 2 diabetics for 12 weeks and also found no difference in HbA1c between the groups. However, a meta-analysis including interventions that ranged in duration between 1 and 12 months found that low-GI and low-GL diets had 0·3–0·5 % greater reductions in HbA1c than those observed for higher-GI or higher-GL alternatives( Reference Thomas and Elliott 13 ). HbA1c serves as a marker for average blood glucose levels over the previous months prior to the measurement. The cause of disagreements between these studies and our results are complicated and may be explained by the short time that we monitored HbA1c. The short-term intervention (10–12 weeks) in our study may not have allowed sufficient time to observe significant changes in HbA1c. Thus, longer-term monitoring is necessary for determining the efficacy of the nutrition advice.
We found that the intake of total energy assessed by the FFQ at baseline was more than 10 MJ/d in our participants, exceeding the amount (8·8 MJ/d) recommended by Chinese dietary reference intakes. In addition to the high energy intake, the greater intakes of carbohydrate and fat than recommended by the dietary reference intakes might partially explain the development of impaired insulin sensitivity, as suggested by recent epidemiological studies( Reference Saldana, Siega-Riz and Adair 32 ). The present study found that general dietary intervention might have a positive impact on the dietary behaviours of individuals. In both the Control and the Low-GL groups, the dietary intakes of energy, fat and carbohydrate decreased significantly after the intervention. Moreover, the Low-GL group outperformed the Control group in reducing the intake of dietary GI and GL and in increasing dietary fibre. A previous Asian trial also documented similar improvements in dietary quality (increasing intake of dietary fibre) in the low-GI arm as compared with conventional recommendations in patients with type 2 diabetes mellitus( Reference Barakatun Nisak, Ruzita and Norimah 33 ).
We also examined the effects of the low-GL dietary intervention on maternal–fetal outcomes and found similar effects on total weight gain and its average value per week and the birth weight of infants compared with the general dietary intervention. Our findings suggested that the low-GL dietary intervention did not decrease fetal growth and did not increase the incidence of adverse pregnant events (e.g. preterm delivery, macrosomia, intra-uterine asphyxia and postpartum haemorrhage). The present results are in accordance with the findings of another RCT, which investigated the effects of a low-GI diet on pregnancy outcomes in GDM( Reference Louie, Markovic and Perera 34 ). The present study found that although the low-GI diet achieved a modestly lower GI, there was no significant difference in birth weight or the prevalence of macrosomia and adverse pregnancy outcomes. However, it should be acknowledged that we did not have sufficient power to detect a difference in the risk of these adverse pregnant events due to limited study size.
Although the validity and reproducibility of the FFQ has been addressed for the participants( Reference Zhang and Ho 27 ), the GI/GL of the FFQ was not validated. However, the validity and reproducibility of the food components are closely correlated to each other in an FFQ because these components are calculated using the same food items. In the FFQ, GI/GL has a similar characteristic to those of macronutrients (e.g. carbohydrate, protein, etc.) and energy. It is unlikely that the validity and/or reproducibility are poor for GI/GL when macronutrients and energy have similar and good values of validity (FFQ v. 24 h recalls, r=0·53 to 0·66)( Reference Shu, Yang and Jin 35 ).
The study was a behavioural intervention study rather than a double-blinded RCT. Both the researchers (the dietitians) and the participants could not be blinded to the group status. We did not use allocation concealment, either. However, to minimize researcher biases, the treatment guidelines for both groups were standardized, all involved dietitians received the same training before the interventions, and the involved dietitians and other researchers (gynaecologists and laboratory technicians) were blinded to the assessments of metabolic results and maternal–fetal outcomes. We observed a lower energy intake but greater weight gain after the intervention as compared with the baseline values. Habitual energy intake might be under-reported or underestimated during the intervention period. Finally, we could not differentiate the effects between the low-GI/GL diet and high dietary fibre in our study, because the low GI/GL value may be caused by a high content of dietary fibre in the diets.
Conclusion
In conclusion, the low-GL targeted dietary intervention outperformed the general dietary intervention in glycaemic control and the improvement of blood lipid levels in GDM women. The low-GL intervention is safe in terms of fetal growth.
Acknowledgements
Acknowledgements: The authors thank all the study participants for their contribution. They also thank Professor Yu-Ming Chen, Department of Epidemiology, Sun Yat-sen University of Medical Sciences, China, for the help with the statistical analysis. Financial support: This study was jointly supported by Science and Technology Program of Guangdong Province, China (grant number 2006B36005013). The funder had no role in the design, analysis or writing of this article. Conflict of interest: None. Authorship: W.-J.M. and Z.-H.H. were the principal investigators and data collectors; B.-X.H., B.-H.Q. and Y.-J.Z. participated in recruiting pregnant women, providing dietary advice and the follow-up survey; B.-X.X. presided at laboratory detection; Y.-H.L. was in charge of physical measurements; L.C. carried out data collection; H.-L.Z. was responsible for the study design and writing the manuscript. All authors were responsible for the critical revision of the manuscript. Ethics of human subject participation: The Human Research Ethics Committee of Guangdong General Hospital approved all procedures (number 2009104H).









