Atherosclerotic vascular disease is the underlying cause of CVD with high morbidity and mortality rates( 1 ). Although the pathophysiological mechanisms underlying atherosclerosis (AS) are not completely understood, it is widely accepted that both oxidative stress and inflammatory stress are key processes involved in the onset and progression of endothelial dysfunction in AS leading to CVD( Reference Badimon and Vilahur 2 , Reference Hansson 3 ). A number of markers that describe oxidative stress and chronic subclinical inflammation at different stages in initiation and progression have been proposed as significant predictors of AS( Reference Dadu, Nambi and Ballantyne 4 , Reference Fruchart, Nierman and Stroes 5 ). Enhanced generation of harmful reactive oxygen species (ROS) induced by either dysregulated antioxidant enzyme systems or deficiency of antioxidative molecules is associated with oxidative stress leading to the oxidation of target molecules to oxidant-modified molecules. As a consequence, the recruitment and adhesion of monocytes is enhanced; for example, oxidant LDL can increase the secretion of soluble chemotactic molecules such as monocyte chemoattractant protein-1 and IL-8 and enhance the expression of cell adhesion molecules (CAM). The expression of integrins such as intracellular adhesion molecule-1 (ICAM-1) and vascular adhesion molecule-1 (VCAM-1), as well as selectins such as E-selectin, has been found to be enhanced on the surface of activated endothelial cells( Reference Badimon, Storey and Vilahur 6 ). The progression is further mediated by the transformation of monocytes into foam cells, which in turn secrete ROS and pro-inflammatory cytokines that maintain the chemotactic stimulus for monocytes adhered to the stimulated vascular endothelium. Evidence regarding the linkage between oxidative and inflammatory stress is also derived from studies with at-risk patients. Deficiency of antioxidant enzyme systems promotes oxidative stress (malondialdehyde (MDA) and 8-iso-PGF2α) and inflammation (ICAM-1 and E-selectin) leading to endothelial dysfunction( Reference Ishigami, Isoda and Adachi 7 , Reference de la Sierra and Larrousse 8 ). Moreover, deficiency of superoxide dismutase (SOD) could promote inflammation by enhancing the secretion of TNF-α and adhesion molecules; administration of SOD3 has been found to alleviate Th2-cell-mediated ovalbumin-induced inflammation in mice( Reference Laurila, Laatikainen and Castellone 9 – Reference Schaller, Dittrich and Felizeter 11 ). Strategies targeting oxidative as well as inflammatory events in the onset of AS and thus preventing the onset of CVD are still being discussed. Besides other plant compounds, the ingestion of anthocyanins (ACN) present in high amounts in red- and purple-coloured fruits could be a beneficial strategy( Reference Siasos, Tousoulis and Tsigkou 12 ). Numerous in vitro cell and in vivo animal studies have confirmed the high potential of ACN to act as direct ROS scavengers through their polyhydroxylated groups or indirectly by influencing the expression and activities of antioxidant enzyme systems such as SOD and catalase (CAT), thus providing evidence that ACN can act as health-promoting agents( Reference Kim, Chung and Ha 13 , Reference Alvarez-Suarez, Dekanski and Ristić 14 ). Recently, the association of the structural characteristics of ACN and their antioxidant capacities with the inhibitory effects on endothelial dysfunction has been established in vitro ( Reference Yi, Chen and Jin 15 ). For example, ACN such as delphinidin exhibit higher ROS-scavenging activities and exert more significant endothelium-protective effects compared with other flavonoids. They can affect the ROS-sensitive NF-κB signalling pathway responsible for the up-regulation of inflammatory cytokine subsets (IL-1β and TNF-α), acute-phase proteins or CAM. Furthermore, it has been shown that a variety of plant extracts from raspberries, blueberries, blackcurrants and strawberries at physiological concentrations induce the basal NF-κB activity as well as NF-κB activation( Reference Paur, Austenaa and Blomhoff 16 ). It has recently been shown that nuclear factor erythroid 2-related factor 2 (Nrf2), together with the ROS-sensitive NF-κB signalling pathway, is an essential transcription factor that regulates the expression of several antioxidant enzymes including SOD and haem oxygenase-1 by binding to the antioxidant response element (ARE)( Reference Nguyen, Nioi and Pickett 17 , Reference Lee and Johnson 18 ). Similar to their possible interaction with the NF-κB pathway, ACN have been shown to play a crucial role in the activation of the Nrf2–ARE pathway. It has been shown that serum-enriched ACN and/or metabolites formed upon the ingestion of ACN induce a significant nuclear accumulation of Nrf2, as well as the expression of Nrf2-regulated antioxidant and cytoprotective genes( Reference Cimino, Speciale and Anwar 19 ). Interestingly, it has recently been shown that the metabolites rather than the parental ACN are responsible for this effect. Phloroglucinol aldehyde, which is known to be a colonic degradation product of ACN, has been shown to significantly increase ARE promoter activity with a concomitant increase in the transcript levels of Nrf2 or haem oxygenase-1( Reference Boettler, Sommerfeld and Volz 20 ).
In spite of the favourable health-promoting effects of ACN, human data are limited and non-distinctive. In some studies, ACN have been identified as antioxidative and anti-inflammatory molecules due to their potential to suppress the secretion of CAM or chemoattractant cytokines (IL-8 and IL-6)( Reference Dohadwala, Holbrook and Hamburg 21 – Reference Kolehmainen, Mykkänen and Kirjavainen 24 ), and in other studies, a significant increase in the secretion of TNF-α has been observed after blueberry consumption( Reference de Mello, Schwab and Kolehmainen 25 , Reference Karlsen, Paur and Bøhn 26 ). As strategies to prevent the onset of AS should be implemented as early as possible, a cross-over intervention study was carried out with young and healthy females to investigate the effects of the ingestion of ACN-rich grape/bilberry juice and smoothie on antioxidative enzymatic and non-enzymatic parameters as well as on cytokine profiles and anti-inflammatory parameters in comparison with those of the ingestion of an ACN-depleted juice as a placebo control.
Subjects and methods
Study design and study subjects
A randomised, double-blind, placebo-controlled, cross-over study was conducted to investigate the effects of ACN-rich beverage ingestion in healthy young individuals. The ANTHONIA (ANTHOcyanins in Nutrition Investigation Alliance) study was carried out at the Institute of Nutritional Science, Justus-Liebig-University Giessen (Germany), between April 2010 and August 2010. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki, and the local ethics committee (registration no. 13/10) approved all procedures involving human subjects. Written informed consent was obtained from all subjects. Of the forty-five healthy female students who volunteered, thirty were deemed eligible for the study according to the specific inclusion criteria (3 months without using medications (drugs and antibiotics) and vitamin and mineral supplementation and free from intestinal diseases and CVD). The subjects included in the study were aged between 23 and 27 years (mean 24·6 (sd 1·2) years); their weight ranged between 44 and 78 kg (mean 59·4 (sd 8·0) kg) and their BMI between 18·2 and 27·9 kg/m2 (mean 21·2 (sd 2·3) kg/m2). To avoid carry-over effects according to permuted-block randomisation, six groups receiving beverages ((1) placebo, (2) juice and (3) smoothie) in different sequences were generated: 1-2-3; 2-3-1; 3-2-1; 3-1-2; 1-3-2; 2-1-3. The subjects were assigned in a double-blind fashion to the initial arm of the study of juice, smoothie or placebo intervention. After randomisation, a 10 d washout period was followed by a 14 d intervention period and a 4 d run-out period. The study design is shown in Fig. 1. After an overnight fast, the subjects consumed 0·33 litres of a beverage (juice, smoothie or placebo) daily with breakfast during the intervention period. They were instructed to keep the beverages cool and to avoid exposing them to direct light. Beverage ingestion was monitored by questionnaires after the follow-up visit (24 h after ingestion) to ensure uptake compliance.

Fig. 1 Design of the ANTHONIA (ANTHOcyanins in Nutrition Investigation Alliance) study. The study participants (n 30) were randomly assigned to three beverage intervention groups. They passed three cycles of the intervention (14 d) receiving fruit juice (juice 1), smoothie (juice 2) or placebo (juice 3). Intervention periods were separated by a 4 d run-out phase and a 10 d washout phase. Before (day 0) and after (day 14) the intervention, blood and 24 h urine samples were collected and processed for biochemical analyses. After a 14 d intervention, the participants crossed over to the alternate beverage. All participants completed the study.
Special care was taken to avoid a possible effect of other phenolics in the diet. As phenolic compounds are present in many foodstuffs and beverages, the participants were explicitly counselled before the start of the study to follow a low-phenolic diet during the washout and intervention periods and maintain their usual physical activity. In addition, they were given a list categorising foodstuffs, including beverages, into ‘not being allowed’, ‘quantitatively limited’ and ‘allowed’. Foodstuffs were categorised according to their ACN content based on data from the USDA Database for the Flavonoid Content of Selected Foods (Release 3.1 (December 2013); http://www.ars.usda.gov/) or the database on polyphenol contents in foods (Polyphenol-Explorer, Release 3.0 (http://www.phenol-explorer.eu/compounds); Table 1). The participants were instructed to keep daily dietary records throughout the study, not only to document their daily dietary intake, but also to ascertain their compliance with dietary instructions. Food intake estimated from daily dietary records was analysed using the DGE-PC professional software (version 4.1.0.043). The baseline characteristics of the study population are given in Table 2.
Table 1 Foodstuffs that were allowed, restricted or not allowed during the washout period

Table 2 Baseline characteristics of the study population* (Mean values and standard deviations, n 30)
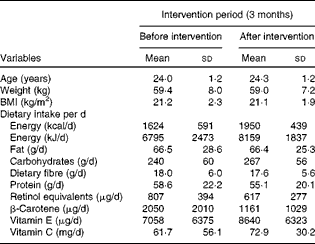
* Dietary records were analysed using the DGE-PC professional software (version 4.1.0.043).
Source of anthocyanin-rich (juice and smoothie) and anthocyanin-depleted (placebo) beverages
Juice, smoothie and placebo juice were produced at the Geisenheim Research Center (Section of Wine Analysis and Beverage Technology) and prepared from an 80:20 mixture of red grapes (grape varieties Dakapo and Accent) and bilberries (Vaccinium myrtillus). Briefly, grapes were extracted in a press, and the resulting juice was separated, blended with bilberry juice from a concentrate, pasteurised and hot-filled into 0·33-litre brown glass bottles. The smoothie was prepared by blending the juice with 20 % bilberry purée instead of bilberry juice. The placebo juice was obtained by passing the grape juice through SP70 absorber resin (Resindion S.r.l.). Juice and placebo juice samples were analysed directly after membrane filtration (0·45 μm) for basic analytical parameters such as concentrations of total phenolics, concentrations of ACN and Trolox equivalent antioxidant capacity (TEAC) as described elsewhere( Reference Fröhling, Patz and Dietrich 27 , Reference Spormann, Albert and Rath 28 ). Smoothie samples were extracted twice for HPLC and TEAC analyses with 80 % methanol. Using 10 ml of 80 % methanol, 5 g of smoothie samples were extracted twice ultrasound-assisted for 30 min. Supernatants were collected and mixed with 80 % methanol after the second extraction. The samples had a pH of 3, so the extraction solution was not acidified. Chromatographic analysis of ACN in juice and placebo juice samples was performed via HPLC/photodiode array detection/electrospray ionisation MS (HPLC–PDA/ESI-MS) using a Thermo Finnigan Surveyor HPLC system coupled to a Thermo Finnigan LCQ Advantage Max mass spectrometer equipped with an electrospray ionisation source and an ion trap mass analyser. For ACN analysis, the mass spectrometer was operated in the positive mode. Chromatographic separation was achieved on a Reprosil Pur 120 ODS-3 column (125 × 2 mm inner diameter, 5 μm; Dr. Maisch GmbH) at a flow rate of 0·2 ml/min at 40°C. Water–formic acid (95:5, v/v) as solvent A and methanol as solvent B were used at a linear gradient from 10 to 40 % B in 18 min. The composition of the ACN-depleted placebo juice and the ACN-rich juice and smoothie is given in Table 3.
Table 3 Composition of the anthocyanin-depleted placebo and anthocyanin-rich beverages (juice and smoothie)* (Mean values and standard deviations, n 2)

ND, not detectable; TEAC, Trolox equivalent antioxidant capacity.
* Juice samples were analysed for standard parameters: TEAC and vitamin C (reductometrically); anthocyanins (HPLC–photodiode array detection/electrospray ionisation MS); total phenolics (Folin reaction, using (+)-catechin as a standard). More details are given in the ‘Materials and methods’ section.
All beverages were matched for fructose content, as it is known to be a confounding variable related to the plasma antioxidant capacity. Beverages were not matched for vitamin C content as it is low, which has been shown to not significantly affect plasma vitamin levels( Reference Spormann, Albert and Rath 28 – Reference Duthie, Jenkinson and Crozier 31 ). ACN were analysed using HPLC–PDA/ESI-MS, and their content is summarised in Table 4. Quantification was carried out in duplicate using peak areas detected at 520 nm and based on external calibration using the reference substance malvidin-3-glucoside (0·1–100 mg/l; linearity of calibration, r 2 0·9999). For malvidin-3-glucoside, the limit of detection was 0·01 mg/l and the limit of quantification was 0·04 mg/l. The ACN analysis was carried out in duplicate.
Table 4 Anthocyanins present in the beverages (anthocyanin-depleted placebo, anthocyanin-rich juice and smoothie)* (Mean values and standard deviations, n 2)

ND, not detectable.
* Beverages were analysed by HPLC–photodiode array detection/electrospray ionisation MS. More details are given in the ‘Materials and methods’ section.
Chemicals
All chemicals were of analytical grade: TCA (ACS reagent, ≥ 99 %), thiourea (puriss. p.a. (purrissimum pro analysis), ACS reagent, ≥ 99·0 %), Cu(II) sulphate hydrate (98 %), 2,4-dinitrophenylhydrazine (puriss. p.a., ≥ 99·0 % (HPLC)), and water CHROMASOLV® (for HPLC) were obtained from Sigma-Aldrich and sulphuric acid (98 %) was obtained from Carl Roth GmbH & Co KG.
Measurement of plasma oxidative and inflammatory biomarkers
Before (day 0) and after (day 14) the intervention, blood and 24 h urine samples were collected to measure oxidative biomarkers (SOD, CAT, glutathione peroxidase (GPx), TEAC, MDA and 8-OH-2-deoxyguanosine (8-OH-dG)) and plasma inflammatory biomarkers (cytokines (IL-2, IL-6, IL-10 and TNF-α), chemokines (IL-8 and monocyte chemoattractant protein-1), CAM (soluble cluster of differentiation 40 ligand, soluble VCAM-1 and soluble ICAM-1) and acute-phase proteins (C-reactive peptide; CRP)). Blood samples were drawn by venepuncture into tubes with EDTA anticoagulant (Sarstedt AG & Co.). Blood samples collected 5 h after juice ingestion were immediately centrifuged (1200 g for 15 min at 4°C) to separate plasma, and the supernatant was divided into aliquots and stored in plastic tubes at − 80°C until analysis. For the determination of erythrocyte SOD activity, plasma was removed and then erythrocytes were haemolysed by addition of an equal volume of ice-cold water (1:1, v/v) and mixed well. After centrifugation for 5 min at 1500 g , lysates were stored at − 80°C. These haemolysates were diluted to 1:20 for the assessment of erythrocyte SOD activity. Erythrocyte SOD activity was assessed as protein concentration in U/mg protein. Protein concentration was determined photometrically using the Pierce™ BCA Protein Assay Kit according to the manufacturer's instructions (Thermo Fisher Scientific).
SOD, CAT and GPx activities were determined by colorimetric methods according to the manufacturer's instructions (Cayman Chemical Company) in a microplate reader (DigiScan 340 and 400; Asys Hitech GmbH).
The amount of water and lipid-soluble antioxidants in plasma was determined using the ABTS (2,2′-azino-di-3-ethylbenzthiazoline sulphonate) assay (Cayman Chemical Company). The generation of ABTS+ from ABTS by metmyoglobin is inhibited by antioxidants, resulting in the reduction of absorbance at 405 nm. The capacity of antioxidants to reduce ABTS+ generation was compared with that of Trolox and quantified as Trolox equivalents (mmol/l TEAC)( Reference Miller and Rice-Evans 32 – Reference Rice-Evans and Miller 34 ).
The measurement of lipid peroxidation levels in plasma is based on the fact that MDA, a naturally occurring product of lipid peroxidation, reacts with thiobarbituric acid under high temperature and acidic conditions (Cayman Chemical Company). The resulting adducts (MDA–thiobarbituric acid) can be determined fluorimetrically (excitation 530 nm/emission 550 nm). The concentration of MDA was calculated using a standard curve, and it is expressed as μmol/ml TBARS (thiobarbituric acid-reactive substances) in plasma or μmol/g creatinine in urine.
The concentration of 8-OH-dG as a marker of oxidative DNA damage induced by ROS was determined using a competitive enzyme-linked sandwich immunoassay (Cayman Chemical Company) for free 8-OH-dG. Concentrations in urine were normalised to those of creatinine and are expressed as pg/g creatinine.
Urinary creatinine concentrations were determined by the Jaffé reaction according to the manufacturer's instructions (http://www.rndsystems.com/pdf/KGE005.pdf). The colour intensity of the creatinine–picrate complex at 490 nm corresponds to the concentration of creatinine in the sample (R&D Systems)( Reference Helger, Rindfrey and Hilgenfeldt 35 – Reference Seelig and Wüst 37 ).
The plasma concentrations of soluble ICAM, soluble VCAM-1, soluble E-selectin, IL-2, IL-6, IL-8, IL-10, TNF-α, high-sensitive CRP, soluble CD40 ligand (sCD40L) and monocyte chemoattractant protein-1 were measured using commercial ELISA according to the manufacturer's instructions (R&D Systems).
The plasma concentration of vitamin C was measured spectrophotometrically according to the method of Lowry et al. ( Reference Lowry, Lopez and Bessey 38 – Reference Watzl, Kulling and Möseneder 40 ). Briefly, the plasma concentrations of total vitamin C (sum of ascorbic acid and dehydroascorbic acid) were measured after protein precipitation. Duplicate aliquots of supernatant were added to a 2,4-dinitrophenylhydrazone/thiourea/CuSO4 solution (50 mm-thiourea, 2 mm-CuSO4 and 150 mm-dinitrophenylhydrazine in 9 m-H2SO4). The dehydroascorbic acid formed from the oxidation of ascorbic acid by Cu was quantified spectrophotometrically as the 2,4-dinitrophenylhydrazone derivative at 550 nm. Ascorbic acid standards were prepared in 5 % TCA, and results are expressed as mg/l.
Statistical analyses
Data obtained from participants who completed all phases of the study protocol (n 30) were analysed. All analyses were carried out in duplicate and intra-assay coefficients of assays were below 10 %. The outcome was evaluated according to the differences in antioxidative and anti-inflammatory parameters of the placebo, juice and smoothie treatment groups before (day 0) and after (day 14) the intervention. The data were also tested for covariates (age, height, weight, BMI, vegetarian, smoking and plasma vitamin C concentrations) using a univariate general linear model (ANCOVA). If significant interactions were observed, data obtained before or after the intervention were analysed using two-way repeated-measures ANOVA models. If no significant effect was observed, before-treatment v. after-treatment data of the groups were analysed using two-tailed paired t test and data that were not normally distributed were analysed using Wilcoxon's signed-rank test. The normality of continuous variables was assessed using the Kolmogorov–Smirnov normality test with Lilliefors correction with a P value < 0·05. Rank or log transformations were performed on some variables before analysis to meet parametric analysis assumptions. Data are expressed as means and standard deviations or as medians and interquartile ranges (IQR; 25th–75th percentiles). Although the study design reduced carry-over effects, the possible carry-over effect was determined by testing a period × treatment interaction term in the general linear models. If no effects were observed, the different intervention periods would be seen independent. The statistical IBM software SPSS for Windows (version 19.0.0) was used for data analysis.
Results
ACN-rich beverages in two different preparations (juice and smoothie) were given to healthy female volunteers over an intervention period of 14 d to investigate the possible effects of food matrix on the antioxidant and anti-inflammatory parameters of the beverages. An ACN-depleted placebo juice was also used to eliminate the effect of other compounds in the beverages according to the study protocol shown in Fig. 1. The baseline characteristics of the study population are summarised in Table 2. No significant changes were observed during the whole study period.
Antioxidant capacity and beverage composition
As shown in Tables 3 and 4, the ACN-rich fruit beverages had a very high antioxidant activity (TEAC value) and a high concentration of total phenolics and ACN in comparison with the ACN-depleted juice. The beverages differed in their polyphenolic composition, which is summarised in Table 4. Malvidin-3-glucoside and peonidin-3-glucoside were identified as the most prominent ACN followed by petunidin-3-glucoside, malvidin-3-(6′-O-acetyl)-glucoside and cyanidin-3-glucoside. In comparison with the ACN-rich beverages, the ACN-depleted placebo juice contained only minor amounts of the ACN listed in Table 4. Because ascorbic acid was present only in moderate amounts (mean < 118 (sd 1) mg/l), it did not seem to make a major contribution to the antioxidant properties of the study juice. The mean plasma vitamin C value at the beginning of the study was 11·8 (sd 2·7) mg/l and did not significantly differ from that recorded at the end of the study (mean 11·3 (sd 2·7) mg/l).
Effect of anthocyanin-rich beverages on antioxidative biomarkers in plasma, erythrocytes and urine
The ingestion of ACN-rich beverages improved the antioxidative status of the participants. Fig. 2 shows the activities of antioxidant enzymes such as SOD, CAT and GPx in plasma and erythrocytes. A significant increase in plasma SOD activity of about 6 % (median 12·95 (IQR 9·71–16·17) v. 13·73 (IQR 11·95–19·65)) after 14 d of juice intervention or of about 21 % (median 13·17 (IQR 9·88–16·46) v. 15·98 (IQR 11·94–18·88)) after smoothie intervention was observed, whereas no change was observed after placebo juice intervention (median 13·78 (IQR 10·33–17·23) v. 13·64 (IQR 10·64–17·01); Fig. 2(a)). In contrast to plasma SOD activity, erythrocyte SOD activity was not affected by 14 d of juice, smoothie or placebo intervention (Fig. 2(b)). Similar to plasma SOD activity, plasma CAT activity was significantly increased after the ingestion of ACN-rich beverages (median 4·29 (IQR 3·22–5·36) v. 5·05 (IQR 3·76–6·31) (P< 0·001) after juice ingestion and median 4·31 (IQR 3·23–5·39) v. 5·33 (IQR 3·40–6·66) (P< 0·001) after smoothie ingestion). After 14 d of placebo juice intervention, CAT activity remained unchanged (Fig. 2(c)). In contrast to the observations for SOD and CAT activities, no significant differences were found in plasma GPx activity after the ingestion of both ACN-rich juice and smoothie (Fig. 2(d)).

Fig. 2 Effects of beverage (placebo, juice and smoothie) intake on antioxidant enzymes before and after the intervention (n 30). The study participants consumed 330 ml of placebo, juice and smoothie over 14 d. Before (0 d) and after (14 d) the intervention, blood samples were drawn and activities of (a) superoxide dismutase (SOD)plasma, (b) SODerythrocytes, (c) catalase (CAT) and (d) glutathione peroxidase (GPx) were measured in plasma or erythrocytes. Values are medians, with 25th to 75th percentiles represented by vertical bars. *** Median value was significantly different from that at baseline (P< 0·001; ANOVA, GLM (general linear mixed model)).
The antioxidant capacity of plasma samples was estimated as TEAC comprising the antioxidative capacity of both lipophilic and hydrophilic compounds (Table 5). When comparing the median TEAC values of the placebo group with those of the ACN-rich smoothie and juice groups, an increase of approximately 10 % from 2·18 (IQR 1·64–2·73) to 2·48 (IQR 1·86–3·10) mmol/ml (P< 0·001) was observed after 14 d of daily juice intervention. After smoothie intervention, the increase in TEAC values was found to be in a similar range (median 2·20 (IQR 1·65–2·75) to 2·43 (IQR 1·82–3·03) mmol/ml; P< 0·01).
Table 5 Effects of beverage (placebo, juice and smoothie) intake on antioxidative parameters before and after the intervention (n 30) (Median values and 25th–75th percentiles)
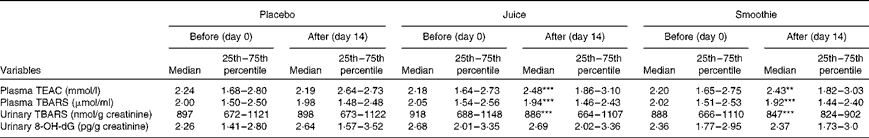
TEAC, Trolox equivalent antioxidant capacity; TBARS, thiobarbituric acid-reactive substances; 8-OH-dG, 8-OH-2-deoxyguanosine.
Median value was significantly different from that at baseline: ** P< 0·01, *** P< 0·001 (ANOVA, GLM (general linear mixed model)).
Lipid peroxidation levels were determined via the formation of TBARS in plasma and urine after 14 d of placebo, juice and smoothie interventions (Table 5). A significant decrease was observed in plasma and urinary TBARS levels after the ingestion of both ACN-rich juice (P< 0·001) and ACN-rich smoothie (P< 0·001). In both cases, the reduction was in a similar range (4 and 6 %, respectively). In addition, no changes were observed in urinary 8-OH-dG concentrations, either on ingesting the juice (P= 0·178) or smoothie (P= 0·285) or on ingesting the placebo juice (P= 0·395).
Effect of anthocyanin-rich beverages on anti-inflammatory biomarkers in plasma
We aimed to investigate whether the ingestion of ACN-rich beverages had an effect on inflammatory parameters. Table 6 summarises the plasma concentrations of cytokines and soluble CAM at baseline and after 14 d of juice intervention. Independent of the kind of intervention used and in contrast to the antioxidative effects, inflammatory biomarkers in plasma were found to remain unchanged throughout the study.
Table 6 Effects of beverage (placebo, juice and smoothie) intake on inflammatory parameters before and after the intervention (n 30) (Median values and 25th–75th percentiles)

MCP-1, monocyte chemoattractant protein-1; hs-CRP, high-sensitivity C-reactive peptide; sCD40, soluble cluster of differentiation 40; sICAM-1, soluble intracellular cell adhesion molecule; sVCAM-1, soluble vascular cell adhesion molecule.
Discussion
Data from human studies concerning the potential of ACN to reduce the incidence of oxidation- and inflammation-related diseases are very limited and investigations in terms of atherogenesis have just begun. We aimed to investigate whether the ingestion of ACN-rich beverages in two preparations (juice and smoothie) would influence the antioxidative parameters and peripheral markers of endothelial cell activation associated with inflammation in a double-blind, placebo-controlled, cross-over intervention study in healthy young females. The major finding of the present study was that the daily intake of about 277·5 mg (juice) or 324·6 mg (smoothie) of ACN from a mixture of red grape juice and bilberry juice or purée (80:20) over 14 d improved the antioxidative status of the volunteers determined by means of both non-enzymatic and enzymatic parameters, independently of the juice preparation. The plasma antioxidant capacity, measured as TEAC, was significantly increased when compared with that observed in the placebo group concomitant with reduced plasma and urinary TBARS levels, but there were no effects on urinary 8-OH-dG concentrations. Published data concerning an improvement of antioxidant activity with concomitant effects on target molecules after ACN ingestion are scarce and inconsistent, not least because of the differences in intervention durations and ingested amounts of ACN from juices or berries and in food matrices as well as due to the presence of other polyphenols( Reference Del Rio, Borges and Crozier 41 ). Although cyanidin-3-glucoside was the major ACN investigated in most studies, the juice or smoothie preparations that we used contained 80 % red grapes and 20 % bilberries with malvidin-3-glucsoide and peonidin-3-glucoside as the major ACN. In comparison with cyanidin-3-glucoside, both these ACN are methylated. Although methylated ACN have a reduced antioxidant capacity in vitro, increased vasodilatation observed after the ingestion of a fruit/vegetable juice could be attributed to methylated flavonoid metabolites( Reference George, Waroonphan and Niwat 42 , Reference Kuhnle, Spencer and Schroeter 43 ). On the other hand, using different methods for assessing the effects of ACN on target molecules could be a further point of interest. Weisel et al. ( Reference Weisel, Baum and Eisenbrand 30 ) reported decreased oxidative DNA damage in peripheral blood mononuclear cells after the consumption of an ACN/polyphenol-rich fruit juice. Although the TEAC (13·9 mm) and ACN concentrations (197·9 mg/l) in the fruit juice were lower than those in the present study, the authors used the highly sensitive comet assay and determined reduced DNA damage even after a short-term intervention of only 1 week. However, they did not observe any changes in MDA concentrations during the 9-week intervention and assumed time-dependent reduction in the levels of lipid peroxidation markers.
Furthermore, it has to be mentioned that it is difficult to correlate antioxidative capacity with serum concentrations of parent ACN. Although intact ACN and their phase II conjugates (glucuronides or sulphates) might be present in the circulation, breakdown products from colonic fermentation (protocatechuic, hippuric, phenylacetic and phenyl propenoic acids) as well as conjugates of the breakdown products (protocatechuic glucuronide and protocatechuic sulphate) are present at much higher concentrations( Reference Koli, Erlund and Jula 44 , Reference Harini and Pugalendi 45 ). Using a 13C tracer study with a single bolus of 500 mg labelled cyanidin-3-glucoside, Czank et al. ( Reference Czank, Cassidy and Zhang 46 ) showed a 42-fold higher abundance of 13C-labelled metabolites relative to [13C]cyanidin-3-glucoside at their maximum serum concentrations. Many of these metabolites also accumulated in the urine or were found in faeces. However, these metabolites could also exert antioxidative effects. In the present study, blood samples were drawn approximately 5 h after juice ingestion with malvidin-3-glucoside and peonidin-3-glucoside being found to be the most prominent circulating ACN. However, we could not conclude that the observed effects were only due to ACN. Using in vitro models, it has been revealed that syringic acid and 2,4,6-trihydroxybenzaldehyde are the breakdown products of malvidin( Reference Forester and Waterhouse 47 ) and malvidin and vanillic acid are the breakdown products of peonidin( Reference Labib, Erb and Kraus 48 ). Furthermore, concomitant with the results reported by Czank et al. ( Reference Czank, Cassidy and Zhang 46 ) using isotope-labelled cya-3-glucoside, numerous phenolic acids have been identified as the in vivo metabolites of ACN following blueberry consumption. For example, protocatechuic acid, a possible breakdown product of cyanidin-3-glucoside( Reference Vitaglione, Donnarumma and Napolitano 49 ), has been found to function as an antioxidant, not only due to its radical-scavenging effects but also due to its ability to influence antioxidant enzyme systems. It has been shown that increased GPx and glutathione reductase expression is induced by c-Jun NH2-terminal kinase-mediated phosphorylation of Nrf2( Reference Varì, D'Archivio and Filesi 50 – Reference Masella, Santangelo and D'Archivio 52 ). In another study using oral supplementation of Medox® (Medox USA), a standardised ACN-rich supplement, plasma ACN concentrations were found to be high enough to affect the Nrf2–ARE pathways( Reference Cimino, Speciale and Anwar 19 ). However, in the present study, the observed improvement in antioxidative capacity and reduced plasma and urinary TBARS levels could be a result of enhanced enzyme activities. The application of both juice and smoothie preparations revealed enhanced plasma CAT and SOD activities, but no influence on erythrocyte SOD activity after the 14 d intervention. Modulation of enzyme activity can occur indirectly through the modification of signal pathways as mentioned above or by direct competitive interactions possibly due to binding to the haem moiety or a protein region of CAT( Reference Doronicheva, Yasui and Sakurai 53 ). However, it has been shown that patients with CVD risk have reduced antioxidant enzyme (SOD and CAT) activities in serum with concomitantly enhanced TBARS levels( Reference Kumar, Mukherjee and Senthil 54 ). Furthermore, if plasma SOD (extracellular SOD) activity is approximately 10-fold higher than cellular erythrocyte CuZn-SOD( Reference Karlsson and Markl 55 , Reference Strålin, Karlsson and Johansson 56 ) activity, ACN would be able to protect against oxidative stress and thus be beneficial not only for healthy individuals but also for patients at CVD risk. In this context, recently published data showed that the intake of freeze-dried blueberries with 375 mg of ACN for 6 weeks in individuals with an increased cardiovascular risk reduced endogenously oxidised DNA bases and H2O2-induced DNA damage without affecting erythrocyte SOD activity( Reference Riso, Klimis-Zacas and Del Bo' 57 ).
Endothelial dysfunction, besides the oxidative stress hypothesis, with changes in inflammatory parameters is considered to determine the first stages of AS. Emerging data have linked ROS generation with inflammatory markers. Furthermore, independent of other risk factors, low-grade inflammation with slightly enhanced secretion of cytokines or acute-phase proteins is predictive of CVD in healthy subjects( Reference Koenig and Wanner 58 ). In the present study, ACN supplementation was found to have no effects on inflammatory markers in healthy young subjects. Neither inflammatory cytokines such as TNF-α and IL-6 nor adhesion molecules such as integrins and acute-phase proteins (e.g. CRP) were affected under the experimental conditions in the present study. Only a few studies have already investigated the effects in healthy volunteers. Cherry consumption for 28 d was found to result in a decrease in CRP concentrations in healthy aged subjects (45–60 years)( Reference Kelley, Rasooly and Jacob 59 , Reference Kelley, Adkins and Reddy 60 ), and the same effect was observed after the consumption of de-alcoholised red wine after a 20 d intervention( Reference Queipo-Ortuño, Boto-Ordóñez and Murri 61 ). The consumption of Medox® by aged volunteers (40–75 years) for 3 weeks was found to be associated with a decrease in the concentrations of NF-κB-regulated pro-inflammatory chemokines and immunoregulatory cytokines; a follow-up intervention with 330 ml of blueberry juice per d for 4 weeks revealed decreased plasma concentrations of CRP and inflammatory cytokines( Reference Karlsen, Retterstol and Laake 62 ). Also, ingestion of freeze-dried grape powder over 30 d was found to slightly decrease soluble ICAM-1 concentrations with a concomitant decrease in blood pressure levels in patients with the metabolic syndrome( Reference Barona, Aristizabal and Blesso 63 ). The reason why ACN-rich beverages did not exert any effect in the present study could be the length of the intervention (2 weeks), the young age (mean 24·6 (sd 1·2) years) of the study population or the time point of blood sampling. Nevertheless, the time course of prevention strategies seems to be important, but studies with aged patients at risk and ACN are extremely rare. For example, an intake of blueberries (freeze-dried powder) over 6 weeks did not reveal any changes with respect to inflammatory biomarkers (TNF-α and CRP) in obese subjects throughout the study carried out by Stull et al. ( Reference Stull, Cash and Johnson 64 ). The consumption of cranberry juice (100 mg ACN/d, 4 weeks, n 44) by patients with coronary artery risk factors or of grape juice (965 mg total polyphenols, 8 weeks, n 17) by patients with hypertension was not found to alter plasma CRP or soluble ICAM-1 concentrations( Reference Dohadwala, Holbrook and Hamburg 21 ).
Taken together, although AS generates severe diseases primarily affecting middle-aged and old people, atherogenesis begins very early in life( Reference Morrison and Atkinson 65 , Reference Napoli, Infante and Casamassimi 66 ). Therefore, primary prevention strategies should be implemented as early as possible. Thus, an evaluation of the functional capacity of ACN to prevent the onset of AS could be a useful strategy regarding the reduction of CVD risk. The participants of the present study were young, and the observed ACN-induced antioxidative health benefits could be considered useful for the primary prevention of CVD. Comparisons of intervention studies with ACN are difficult due to variations in study designs, ACN patterns, dosages and preparations as well as differences in the polyphenol content of the general diet during the study. Although the observed effects on antioxidative markers can be attributed to ACN, it should be kept in mind that the ACN-rich juice and smoothie contained additional polyphenols such as polymeric ACN and simple phenolic acids such as protocatechuic acid and chlorogenic acid, which have also been shown to have the potential to influence antioxidative and anti-inflammatory parameters in vitro and in vivo ( Reference Shi, Dong and Dang 67 – Reference Volz, Boettler and Winkler 69 ).
The strength of the present study was the homogeneous study population (n 30) and its cross-over design that permitted the same participants to receive all treatments, which minimised interferences of possible confounding variables. Division of the study population into six groups with different treatment sequences minimised carry-over effects. Furthermore, the application of an ACN-depleted placebo juice as a control beverage and dietary restrictions of polyphenol intake without affecting basal activities was a further important point in the study design. A limitation of the study was that the volunteers were healthy without indications of CVD events, which made it difficult to detect an effect on inflammation-associated events in atherogenesis. In further studies, emphasis should be placed on the identification and characterisation of ACN and their metabolites in plasma, which would provide information on metabolites responsible for the effects of ACN. In conclusion, the present study demonstrates that due to their potent antioxidant activities, ingestion of ACN-rich beverages could be a useful strategy to reduce the risk of CVD.
Acknowledgements
The authors cordially thank the study participants. They also thank Cordula Becker, Nadine Metz, Sina Streichert, Nicole Tscherney and Marcel Zoremba for their excellent technical support in performing blood and urine sample analyses.
The present study was funded by the German Ministry of Education and Research (BMBF) as part of the research consortium ANTHONIA (grant no. 0315379A). The BMBF had no role in the design and analysis of the study or in the writing of this article.
The authors' contributions are as follows: S. K., S. R. and C. K. were the principal investigators of the study and responsible for the study design and wrote the manuscript; G. A. and C. H. B. contributed to the study design, intervention and sample handling; B. F. and H. D. prepared the ACN-rich and ACN-depleted beverages and measured ACN content; J. H. was responsible for the statistical analyses. All authors approved the final version of the manuscript.
None of the authors has any conflicts of interest to declare.












