Obesity has become a public health concern due to its positive association with the incidence of CVD, type 2 diabetes mellitus, hypertension, stroke, dyslipidaemia, osteoarthritis and some cancers( Reference Zhang, Sun and Wang 1 ). Therefore, prevention of obesity is a prophylactic measure for many of these diseases.
Coffee beans contain several bioactive components such as chlorogenic acid (CGA) and caffeine. CGA, the ester of caffeic acid and quinic acid, is one of the most abundant polyphenols in the human diet and has been reported to decrease the incidence of chemical carcinogenesis in several animal models of cancer and to suppress the growth of bacteria( Reference Monteiro, Farah and Perrone 2 ). CGA has various biological properties, such as antioxidant, anti-mutagenic, anti-cancer and matrix metalloproteinase-inhibiting properties( Reference Narita and Inoue 3 ). CGA has been claimed not only to delay glucose absorption in the intestine but also to decrease hepatic glucose output through the inhibition of glucose-6-phosphatase activity( Reference Ong, Hsu and Tan 4 ). Cho et al. ( Reference Cho, Jeon and Kim 5 ) have demonstrated that CGA significantly lowers the concentrations of NEFA, TAG and total cholesterol (TC) in the serum. It has also been reported that 0·1 % CGA significantly enhances the BMR of rats fed a high-fat diet( Reference Hirata, Kobayashi and Wada 6 ). Moreover, Hsu et al. ( Reference Hsu, Hung and Yen 7 ) have reported that CGA inhibits the growth of preadipocyte populations. Caffeine ingestion reduces the size of adipose pads and the number of adipocytes( Reference Greenberg, Boozer and Geliebter 8 ) and enhances the anti-inflammatory response( Reference Tauler, Martinez and Moreno 9 ). Recently, it has been demonstrated that caffeine exerts a beneficial effect on adipose-derived stem cells and bone marrow stromal cells by enhancing their differentiation into osteoblasts( Reference Su, Chang and Su 10 ). Zheng et al. ( Reference Zheng, Sayama and Okubo 11 ) have shown that caffeine reduces the weight of intraperitoneal adipose tissues (IPAT) in mice. Yoshioka et al. ( Reference Yoshioka, Yoshida and Kamanaru 12 ) have demonstrated that a high concentration of caffeine (60 mg/kg body weight) significantly increases brown adipose tissue thermogenesis and RMR and that a low concentration (40 mg/kg body weight) has no effects in mice.
These findings demonstrate that a high dose of caffeine has an anti-obesity effect and that CGA is a hypolipidaemic agent. However, studies documenting the effect of these compounds in combination on lipid metabolism are sparse. We hypothesised that a combination of caffeine and CGA might have an inhibitory effect on fat accumulation and obesity development. To test this hypothesis and identify the mechanism by which this might occur, in the present study, we investigated the effects of CGA and caffeine alone and in combination on the body weight, biochemical parameters of the liver, IPAT, and serum, and activities and mRNA and protein expression of lipid metabolism-related enzymes in mice.
Materials and methods
Ethics statement
The present study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of China Association for Laboratory Animal Science. All animal care and use protocols were approved by the Animal Care and Use Committee of Jiangxi Agricultural University. Animals were killed under anaesthesia, and every effort was made to minimise their suffering.
Animals and diets
Female ICR mice aged 4 weeks and weighing about 20 g were purchased from Animal Breeding and Research of Jiangxi Medical College (Jiangxi, China). Caffeine (>99 %) was obtained from Johnson Matthey Company. CGA (>90 %) was purchased from Hunan Liuyang Aite Natural Product Research and Development Company Limited.
All mice were acclimated on a standard AIN-93 G diet for 1 week. Then, forty mice were weight-matched and divided into four groups and fed diets containing no CGA or caffeine, 0·2 % CGA, 0·03 % caffeine, and 0·2 % CGA+0·03 % caffeine. Mice were given free access to food and tap water for 24 weeks. During the feeding period, mice were weighed every week. Mice were anaesthetised and killed after the experiment, and blood was drawn from the heart and allowed to clot at room temperature. Serum was isolated by centrifugation at 825 g for 15 min at 4°C. The liver and IPAT of each mouse were harvested and weighed. Food intake was measured every day for 2 weeks. All mice were housed in an air-conditioned (temperature 24 ± 2°C and humidity 50 ± 10 %) and light-controlled (12 h light–12 h dark cycle, lights on from 08.00 to 20.00 hours) animal room.
Biochemical analysis of serum and hepatic parameters
The serum concentrations of TAG, TC and glucose were determined using commercial kits (Biosino Biotechnology and Science, Inc.). The concentrations of NEFA were determined using a commercial kit (Nanjing Jiancheng Bioengineering Institute). The serum concentration of leptin was measured using ELISA kits (R&D systems). Total lipids in the liver were extracted using the method of Folch et al. ( Reference Folch, Lees and Sloane Stanley 13 ). The hepatic concentrations of TAG and TC were determined using commercial kits (Biosino Biotechnology and Science, Inc.). The concentrations of phospholipids were determined using the method of Bartlett( Reference Bartlett 14 ).
Measurement of the activities of hepatic lipid metabolism-related enzymes
Frozen liver samples were homogenised in buffer A (3 mm-Tris–HCl, pH 7·2, 1 mm-EDTA, 1 mm-dithiothreitol, 25 μm-ALLN (a calpain and cathepsin inhibitor, N-acetyl-leucyl-leucyl-norleucinal), 100 μm-leupeptin, 100 μm-AEBSF (a serine protease inhibitor, 4-(2-aminoethyl)bezenesulphonyl fluoride), 10 μm-E64 and 0·25 m-sucrose) according to the method of Moriyama et al. ( Reference Moriyama, Kishimoto and Nagai 15 ). The protein concentration of the homogenate was measured and then adjusted to 10 mg/ml for carnitine acyltransferase (CAT) activity analysis. The homogenate was centrifuged at 500 g for 10 min and the supernatant was used for acyl-CoA oxidase (ACO) activity analysis. The supernatant (500 g ) was further centrifuged at 9000 g for 15 min, and the resulting supernatant was used for fatty acid synthase (FAS) activity analysis. The activities of CAT, ACO and FAS were determined by the methods of Markwell et al. ( Reference Markwell, McGroarty and Bieber 16 ), Osumi & Hashimoto( Reference Osumi and Hashimoto 17 ) and Kelley et al. ( Reference Kelley, Nelson and Hunt 18 ), respectively.
Measurement of the mRNA expression of lipid metabolism-related enzymes
Total RNA was isolated from frozen liver samples using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. Complementary DNA was obtained by reverse transcription of 1 μg of RNA using the cDNA Reverse Transcription Kit (Tanaka Biological, Inc.) according to the manufacturer's protocol. Real-time quantitative PCR was carried out using the Applied Biosystems 7900HT Real-Time PCR System (Applied Biosystems) with Premix Ex Taq™ (Probe qPCR) according to the manufacturer's protocol. Briefly, PCR was carried out in a final volume of 20 μl containing 800 ng of complementary DNA, 0·4 μl of forward and reverse primers, 0·4 μl of fluorescence probe, 10 μl of Premix Ex Taq and 0·4 μl of ROX Reference Dye. PCR consisted of an initial denaturation step at 95°C for 30 s, followed by forty-five amplification cycles of 10 s at 94°C and 37 s at 60°C. The primers and probes used are given in Table 1. The results are presented as expression levels relative to those of the control after normalisation to glyceraldehyde 3-phosphate dehydrogenase using the 2− ΔΔC T method.
Table 1 Gene-specific primers and probes used in quantitative real-time PCR

ACO, acyl-CoA oxidase; CAT, carnitine acyltransferase; AMPK, AMP-activated protein kinase; ATGL, adipose TAG lipase; GADPH, glyceraldehyde 3-phosphate dehydrogenase.
Measurement of the protein expression of AMP-activated protein kinase, adipose TAG lipase and fatty acid synthase in the liver
Liver tissue samples were ground in liquid N2 and lysed in a radioimmunoprecipitation assay buffer (50 mm-Tris, pH 7·4, 150 mm-NaCl, 1 % Triton X-100, 0·5 % sodium deoxycholate, 0·1 % SDS, 1 mm-EDTA, 1 mm-phenylmethanesulphonyl fluoride and 2 μg/ml leupeptin) at 4°C for 1 h. Liver lysates were centrifuged at 9000 g for 15 min, and the supernatant was used for measuring the protein expression of FAS, AMP-activated protein kinase (AMPK) and adipose TAG lipase (ATGL). The protein concentration of each supernatant was determined. Equal amounts of protein (30 μg) were resolved by SDS–PAGE and transferred onto polyvinylidene difluoride membranes (Millipore). The AMPK, FAS and ATGL blots were blocked with 5 % non-fat dry milk–TBST buffer (TBS containing 0·1 % Tween-20) for 2 h at room temperature. The membranes were rinsed three times for 10 min each with TBST buffer and then incubated overnight at 4°C with 1:1000 dilutions of antibodies against AMPKα (no. 2793; Cell Signaling Technology, Inc.), FAS (no. 3180; Cell Signaling Technology, Inc.) and ATGL (ab85858; Abcam Limited). Equal lane loading was assessed using β-actin (Zhongshan Bio Company Limited). The blots were rinsed three times with TBST buffer for 10 min each. Washed blots were incubated with a 1:1000 dilution of a horseradish peroxidase-conjugated secondary antibody solution (ZSGB-BIO) for 2 h and washed three times with TBST buffer. The transferred proteins were visualised using an enhanced 3,3′-diaminobenzidine tetrahydrochloride kit (ZSGB-BIO).
Statistical analysis
All data are presented as means with their standard errors. Student's t test was used to compare the mean differences after analysis using the Data Processing System software (version 6.55, Hangzhou Reifeng Information Technology Company Limited, China; http://www.dpsw.cn). A P value < 0·05 was considered significant and a P value < 0·01 very significant.
Results
Body weight, organ weights and food intake
The effects of CGA and caffeine on the body weight, liver weight and IPAT weight of mice are summarised in Table 2. A decreasing trend was observed in the body weight of the treatment groups. There was a significant decrease in the body weight gain of mice fed the CGA+caffeine diet (P< 0·05). Furthermore, IPAT weight was significantly reduced in mice fed the CGA+caffeine diet (P< 0·05) when compared with that in the control group. There were no significant differences in food intake between the treatment and control groups (control, CGA, caffeine and CGA+caffeine (g/d per mouse): 4·59 (sem 0·18), 4·48 (sem 0·32), 4·48 (sem 0·47) and 4·62 (sem 0·22), respectively). These results indicate that CGA+caffeine decreased the body weight and IPAT weight of mice with no effects on food intake.
Table 2 Effects of caffeine and chlorogenic acid (CGA) on the body weight, liver weight and intraperitoneal adipose tissue (IPAT) weight of mice (Mean values with their standard errors, n 10)
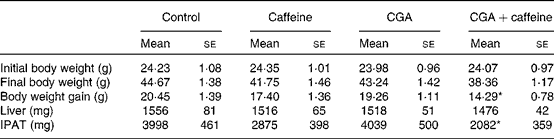
* Mean value was significantly different from that of the control group (P< 0·05).
Serum biochemical parameters and hepatic lipid profiles
The effects of CGA and caffeine on the serum biochemical parameters and hepatic lipid profiles of mice are summarised in Table 3. There was a significant decrease in the serum leptin concentration of mice fed the CGA+caffeine and caffeine diets (P< 0·05) compared with that of the control group. The serum concentrations of TC and TAG were also significantly lower in mice fed the CGA and CGA+caffeine diets than in the control group (P< 0·05). There was a significant decrease in the hepatic TAG concentrations of mice fed the caffeine and CGA+caffeine diets (P< 0·05). These results indicate that CGA+caffeine decreased serum and hepatic lipid levels.
Table 3 Effects of caffeine and chlorogenic acid (CGA) on the serum biochemical parameters and hepatic lipid profiles of mice (Mean values with their standard errors, n 10)
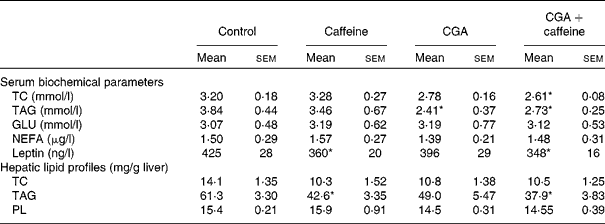
TC, total cholesterol; GLU, glucose; PL, phospholipids.
* Mean value was significantly different from that of the control group (P< 0·05).
Activities of hepatic lipid metabolism-related enzymes
The effects of CGA and caffeine on the activities of CAT, ACO and FAS in the liver of mice are shown in Fig. 1. The activity of CAT was markedly increased in mice fed the CGA+caffeine (P< 0·01) and caffeine (P< 0·05) diets than in the control group. The activity of ACO was significantly increased in mice fed the caffeine and CGA+caffeine diets (P< 0·05), while that of FAS was significantly decreased in mice fed the CGA+caffeine diet (P< 0·05).

Fig. 1 Effects of chlorogenic acid (CGA) and caffeine on the activities of hepatic lipid metabolism-related enzymes. CAT, carnitine acyltransferase; ACO, acyl-CoA oxidase; FAS, fatty acid synthase. Values are means of ten mice, with their standard deviations represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05). □, Control; ![]() , caffeine; ■, CGA;
, caffeine; ■, CGA; ![]() , CGA+caffeine.
, CGA+caffeine.
mRNA expression of lipid metabolism-related enzymes
The mRNA expression levels of lipid metabolism-related enzymes were analysed by real-time quantitative PCR, and the results are shown in Fig. 2. The mRNA expression levels of AMPK, ACO, CAT and ATGL were up-regulated by both caffeine and CGA individually and to a greater extent when used in combination. These results indicate that CGA+caffeine synergistically affected the regulation of these genes. However, the mRNA expression levels of PPARγ2 were significantly down-regulated in mice fed the CGA and CGA+caffeine diets (P< 0·05), while there were no significant differences in the levels between the caffeine diet-fed and control groups.

Fig. 2 Effects of chlorogenic acid (CGA) and caffeine on the hepatic mRNA expression levels of AMP-activated protein kinase (AMPK), carnitine acyltransferase (CAT), acyl-CoA oxidase (ACO), adipose TAG lipase (ATGL) and PPARγ2 analysed by real-time quantitative PCR. Values are means of ten mice, with their standard deviations represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05). □, Control; ![]() , caffeine; ■, CGA;
, caffeine; ■, CGA; ![]() , CGA+caffeine.
, CGA+caffeine.
Protein expression of AMP-activated protein kinase, adipose TAG lipase and fatty acid synthase in the liver
The protein expression levels of AMPK, ATGL and FAS were analysed by Western blotting, and the results are shown in Fig. 3. Compared with that in the control group, there was a decrease in the protein expression levels of FAS in all the treatment groups. In particular, a significant difference was observed between the CGA+caffeine diet-fed and control groups (P< 0·05). There was a significant increase in the protein expression levels of AMPK and ATGL in mice fed the CGA+caffeine diet (P< 0·05).

Fig. 3 Effects of chlorogenic acid (CGA) and caffeine on the hepatic protein expression levels of AMP-activated protein kinase (AMPK), adipose TAG lipase (ATGL) and fatty acid synthase (FAS). (a) Western blot analysis of AMPK, ATGL and FAS proteins isolated from the liver of mice. (b) The intensities of AMPK, ATGL and FAS protein expression levels relative to those of the control after normalisation to β-actin. Values are means of ten mice, with their standard deviations represented by vertical bars. * Mean value was significantly different from that of the control group (P< 0·05). □, Control; ![]() , caffeine; ■, CGA;
, caffeine; ■, CGA; ![]() , CGA+caffeine.
, CGA+caffeine.
Discussion
In the present study, the effects of CGA + caffeine on serum and hepatic lipid levels were investigated. The results clearly showed that CGA+caffeine reduced serum and hepatic lipid levels and thus led to the suppression of body weight gain and fat accumulation in the treated mice mice. Moreover, to investigate the effects of CGA+caffeine on lipid metabolism, the activities of liver metabolism-related enzymes were analysed. The results showed that CGA+caffeine increased the activities of CAT and ACO and suppressed the activity of FAS in the liver. As the liver is actively involved in β-oxidation, stimulation of lipid metabolism may contribute to the suppression of hepatic and visceral fat accumulation( Reference Murase, Nagasawa and Suzuki 19 ). The activity of FAS has been reported to be positively correlated with the amount of body fat( Reference Li, Shi and Tian 20 ). The results of the present study indicate the enhancement of β-oxidation and suppression of lipogenesis to be the major reasons for the reduction of fat accumulation and body weight gain in mice.
CGA has previously been shown to significantly reduce body weight and visceral fat mass in high-fat diet-induced obese mice( Reference Cho, Jeon and Kim 5 , Reference Peng, Liu and Chuang 21 ). In the present study, CGA was found to have no effect on body weight or fat accumulation, which may be due to the normal diet rather than the high-fat diet that was used. Miura et al. ( Reference Miura, Miura and Yagasaki 22 ) have reported that apple polyphenols (e.g. CGA) suppress lipid absorption and increase faecal lipid content. Caffeine has been shown to increase energy expenditure during thermogenic responses( Reference Riedel, Pignitter and Hochkogler 23 ). It has also been shown that caffeine can reduce adipose tissue weight in animals( Reference Zheng, Sayama and Okubo 11 , Reference Yoshioka, Yoshida and Kamanaru 12 ). Moreover, the intake of caffeine has been shown to elevate the serum concentrations of catecholamine in rats fed a high-fat diet( Reference Kobaysahi-Hattori, Mogi and Matsumoto 24 ). Tanaka et al. ( Reference Tanaka, Tamaru and Nishizono 25 ) postulated that caffeine enhances the degradation of fat in adipose tissues by stimulating catecholamine secretion. Some of the fatty acids that are released from adipose tissues are transported to the liver and are then oxidised. Thus, the reduced deposition of visceral fat may be related to the enhanced oxidation of fatty acids in the liver. In the present study, caffeine and CGA+caffeine were found to enhance the activity of CAT in the liver. This increased activity may be responsible for the reduction of adipose tissue weight and the suppression of body weight gain.
Leptin is produced by adipose tissues, and the serum concentrations of leptin are directly proportional to adipose tissue weight( Reference Janeckova 26 ). Therefore, the decrease in leptin concentrations may be attributable to the reduction of IPAT weight in mice fed the CGA and caffeine diets. In mice fed the CGA+caffeine diet, leptin concentrations were significantly decreased and IPAT weight was considerably reduced, supporting the theory that the reduction of IPAT weight is related to the decrease in leptin concentrations. Neither CGA nor caffeine was found to significantly suppress body weight gain or reduce IPAT weight in the present study. However, CGA+caffeine significantly suppressed body weight gain and reduced IPAT weight. It has been shown that CGA may act synergistically with caffeine to produce anti-obesity effects.
CGA has been shown to effectively lower TC and TAG concentrations in the serum and liver( Reference Peng, Liu and Chuang 21 ). In the present study, CGA+caffeine was found to reduce serum and hepatic TC and TAG concentrations. The decrease in TAG concentrations in mice is thought to be induced by both the suppression of fatty acid synthesis and the acceleration of fatty acid oxidation. It has been demonstrated that caffeine elevates the activity of ACO in the liver( Reference Kobaysahi-Hattori, Mogi and Matsumoto 24 ). In the present study, mice were fed caffeine, CGA, and CGA+caffeine diets for 12 weeks. Both the caffeine and CGA+caffeine diets enhanced the activities of CAT and ACO in the liver, which indicates that caffeine can accelerate hepatic lipolysis by increasing the activities of ACT and ACO. In the present study, CGA+caffeine was found to inhibit the activity of FAS and down-regulate the expression of FAS protein. CGA has been shown to inhibit the activity of FAS( Reference Li, Ma and Wu 27 ), and there are a few reports that suggest that caffeine affects the activities of fatty acid synthesis enzymes. Hence, CGA in the CGA+caffeine diet may be responsible for the suppression of FAS activity in the liver.
AMPK plays an important role in the regulation of glucose and lipid metabolism( Reference Lee, Hur and Hwang 28 – Reference Zhang, Zhou and Li 30 ). When activated by conditions that deplete energy such as hypoxia, ischaemia and glucose deprivation, AMPK turns off ATP-consuming processes, such as fatty acid synthesis, cholesterol synthesis and gluconeogenesis, and turns on catabolic pathways that produce ATP, such as β-oxidation, glycolysis and glucose uptake( Reference Lee, Hur and Hwang 28 ). The activation of AMPK leads to numerous metabolic changes, which are potential targets in the treatment of metabolic disorders such as obesity, type 2 diabetes and the metabolic syndrome. In the present study, CGA+caffeine was found to significantly increase the expression of AMPK (P< 0·05), while caffeine or CGA alone was found to lead to upward trends, but these were unremarkable. Interestingly, the mRNA expression levels of AMPK, ACO and CAT were significantly up-regulated by CGA+caffeine, which indicates that the increased expression of AMPK might have promoted the expression of ACO and CAT, which are the main contributors of β-oxidation. To confirm these findings, the expression of AMPK was measured and CGA+caffeine was found to significantly increase the protein expression levels of AMPK. ATGL is highly expressed in white adipose tissue and is the predominant TAG hydrolase in mammals, and although its role in the liver is largely unknown, it does appear to be a major hepatic TAG lipase( Reference Ong, Mashek and Bu 31 ). Together with hormone-sensitive lipase, it accounts for more than 90 % of TAG hydrolase activity in the white adipose tissue of mice( Reference Lord and Brown 32 ). The activity of TAG hydrolase in the white adipose tissue of ATGL− / − mice is 80 % lower than that in the tissue of wild-type mice, suggesting that ATGL is the rate-limiting enzyme in TAG hydrolysis in white adipose tissue, whereas hormone-sensitive lipase functions primarily as a diglyceride hydrolase( Reference Haemmerle, Lass and Zimmermann 33 ). In the present study, the expression of ATGL mRNA was increased by both caffeine and CGA, but CGA+caffeine yielded more dramatic results. Western blotting showed that caffeine, CGA and CGA+caffeine up-regulated the protein expression levels of ATGL considerably, which indicates that CGA+caffeine may contribute to lipolysis. Further investigation is required to determine whether the increased AMPK levels promoted the expression of ATGL. AMPK is activated by two distinct pathways, an AMP-dependent pathway mediated by liver kinase B1 and a Ca2+-dependent pathway mediated by Ca(2+)/CaM-dependent protein kinase kinase-β( Reference Sanders, Grondin and Hegarty 29 ). The activation of AMPK depends on the phosphorylation of Thr172 on its α-subunit, which leads to reduced energy storage and increased energy production to re-establish normal cellular energy balance( Reference Zhang, Zhou and Li 30 ). Binding of AMP to the γ-subunit leads to the allosteric activation of AMPK, as well as to the protection of Thr172 from dephosphorylation, thereby keeping the enzyme activated. Further studies on the relationship between phosphorylated AMPK and ACO, CAT and ATGL are required to fully evaluate this mechanism.
PPARγ is a nuclear receptor with many diverse functions, including the regulation of genes associated with proliferation and differentiation, in a variety of cell types. Its most remarkable function is the regulation of adipose tissue development, which involves coordination of the expression of thousands of genes responsible for the establishment of the mature adipocyte phenotype( Reference Farmer 34 ). Many investigations have demonstrated that PPARγ2 is a potential physiological sensor of lipid levels, linking fatty acids and other lipid-related molecules to glucose and lipid homeostasis. PPARγ2 regulates the expression of adipogenic genes, is expressed selectively in adipose tissues, and promotes the differentiation and proliferation of adipocytes, leading to an increase in adipose tissue mass( Reference Otto, Lane and Cox 35 , Reference Gustafson and Smith 36 ). In the present study, the mRNA expression levels of PPARγ2 were significantly down-regulated in the liver of mice fed the CGA and CGA+caffeine diets, while no remarkable regulating effects were observed in mice fed the caffeine diet, indicating that caffeine enhanced a CGA-mediated decrease in PPARγ2 mRNA expression levels and then contributed to lipolysis. Similar to PPARγ, CCAAT enhancer-binding protein-α is also known to be a key transcription factor for gene activation and differentiation. Both proteins are important for lipogenesis at the molecular level, because they cross-regulate each other's expression as well as govern the expression of the entire adipogenic programme, which includes the activation of additional transcription factors( Reference Wu, Rosen and Brun 37 ).
It might be interesting to equate the doses of caffeine and CGA used in the present study with those found in a typical cup of coffee. This is not entirely straightforward as these values vary dramatically depending on the type of coffee and the preparation method( Reference Niseteo, Komes and Belščak-Cvitanović 38 ). Typical values in green coffee bean are of the order of 8 % for CGA and 1·2 % for caffeine, so the doses used in the present study are not too far removed from the daily consumption levels. The dose of caffeine used in the present study approximately equals that present in two cups of coffee.
In summary, the results of the present study demonstrate that CGA+caffeine affects the activities of lipid metabolism-related enzymes through the regulation of their mRNA and protein expression levels. The enhancement of fatty acid oxidation and suppression of FAS activity were found to decrease serum and hepatic lipid levels and then suppress fat accumulation and body weight gain. The up-regulated AMPK mRNA and protein expression may promote lipolysis. The results also indicate that it might be possible to prevent obesity by continuous and long-term administration of CGA and caffeine together. In light of such beneficial effects, the coffee bean has potential as a functional food that can prevent the onset of lifestyle-related diseases.
Acknowledgements
The present study was supported by the National Natural Science Foundation of China (no. 31160320) and sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholar, State Education Ministry (2009). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors' contributions are as follows: G. Z. conceived and designed the experiments; Y. Q. and D. L. carried out the experiments; G. Z., Q.-F. Z. and D. L. analysed the data, G. Z. and Y. Q. wrote the article.
None of the authors has any conflicts of interest to declare.










