INTRODUCTION
Since the introduction of Haemophilus influenza type B (Hib) and pneumococcal conjugate vaccines, Neisseria meningitidis has become the leading cause of bacterial meningitis and sepsis in the UK and other countries. N. meningitidis can affect otherwise healthy people of all ages; when it occurs, the disease progresses rapidly, even with appropriate medical intervention, and can lead to death within 24–48 h of the first symptoms [Reference Thompson1]. Furthermore, 10–20% of survivors have serious long-term complications such as deafness, limb amputation or neurological deficits [Reference Rosenstein2].
Of the 13 serogroups identified, serogroups A, B, C, W-135, X and Y are responsible for nearly all meningococcal disease worldwide [Reference Girard3, Reference Stephens, Greenwood and Brandtzaeg4]. The incidence of N. meningitidis disease is highest in children aged <2 years and shows a secondary peak in adolescents and young adults in many geographical regions [5]. In contrast, the number of meningococcal carriers is highest in young adults [Reference Christensen6].
Before the introduction of a serogroup C meningococcal (MenC) conjugate vaccine in the UK, about 2400 cases of meningitis were reported annually, predominantly due to serogroups B and C [Reference Health7]. In 1999, a MenC vaccination campaign was implemented with catch-up initially targeting all children and adolescents aged from 2 months to 18 years and subsequently extended to individuals up to 24 years of age. As a result, during the first 2 years of the UK MenC conjugate immunization programme, the attack rate for invasive meningococcal serogroup C disease in the unvaccinated population was reduced by 67% [Reference Ramsay8]. Serogroup C disease rates declined markedly in both immunized and non-immunized individuals, presumably as a result of reductions in carriage and subsequent ‘herd’ protection. The success of the UK MenC programme led to similar introductions in other countries, e.g. Ireland, The Netherlands, Spain, and Australia [Reference Trotter and Ramsay9, Reference Cohen10].
In countries where vaccination programmes comprised a large catch-up campaign, such as the UK and The Netherlands, the decline in disease incidence was more immediate than in those countries without such programmes [11, Reference Kaaijk12].
Following the introduction of MenC, meningococcal serogroup B (MenB) has emerged as the most prevalent serogroup in Europe [13]. In the UK, 84% of meningococcal infections are caused by serogroup B, with an average of 1400 cases annually over the decade from 2001 to 2010 [Reference Health7].
No meningococcal vaccine is currently indicated for prevention of MenB infections; however, a new multi-component vaccine – Bexsero® (Novartis, Switzerland) – was approved by the European Commission in January 2013 for use in the European Union in individuals aged ⩾2 months. Bexsero has four components: outer membrane vesicles (OMVs) from the New Zealand MenB outbreak strain; factor H binding protein; neisserial adhesin A; and Neisseria heparin-binding antigen. In clinical studies, Bexsero has generated protective MenB antibody levels, with acceptable tolerability in subjects as young as 2 months [Reference Gossger14] and protective antibody levels against meningococci-expressing capsular groups other than B (mainly serogroups A, C, W-135, X, Y) [Reference Claus15, Reference Hong16].
The objective of this analysis was to quantify the potential benefits of a Bexsero vaccination programme in the UK for the prevention of meningococcal disease associated with serogroup B and potential benefits vs. serogroups W-135, and Y. Serogroups A and X were not considered in this analysis owing to the current low number of cases in the UK. A transmission model that considers both the direct effects of vaccination and the impact of herd immunity was used to recommend a vaccination schedule that would lead to (1) a rapid reduction in the overall number of vaccine-preventable (VP) meningococcal cases, and (2) long-term elimination of VP meningococcal disease.
METHODS
The model
This dynamic transmission model was based on a published model of meningococcal serogroup C disease transmission [Reference Trotter, Gay and Edmunds17] and adapted to describe the transmission of Bexsero-preventable meningococcal disease. In addition, using recently published carriage data, the model was adapted to include the use of UK-specific population contact matrices for calculating disease transmission and UK population estimates, birth projections and mortality data rather than a uniform population distribution. The model was been programmed using Microsoft Excel 2010 (Microsoft, USA).
The model is dynamic in that the force of infection changes over time based on rates of carriage within the population; further, the rates of carriage change with the force of infection. Using this model structure, it was possible to estimate indirect benefits of the vaccine on the vaccinated and unvaccinated population by simulating the vaccine's impact on carriage acquisition. If a vaccine demonstrates an impact on carriage acquisition, the overall number of subjects carrying the bacteria is reduced within a population, in turn, reducing the risk of infection for susceptible subjects and leading to herd protection.
Structure
The transmission model comprised a set of nine mutually exclusive compartments defined in terms of VP (M) or non-VP (O) meningococcal carriage and vaccination status (Fig. 1). At the starting point, subjects are distributed across compartments based on assumptions regarding the current prevalence of vaccination, infection and disease. The first column of Figure 1 represents seroprotected subjects, the second non-seroprotected subjects, and the third those with meningococcal disease. The top row represents subjects infected with VP meningococcal strains, the second row represents uninfected subjects who are susceptible to infection, and the third row represents subjects infected with non-VP meningococcal strains, for whom it is assumed that co-infection with VP meningococcal disease cannot occur.

Fig. 1 [colour online]. Meningococcal transmission model. M, Infected (vaccine preventable); MR, infected seroprotected (vaccine preventable); OR, infected seroprotected (non-vaccine preventable); O, infected (non-vaccine preventable); S, susceptible; SR, susceptible seroprotected; i, age stratum.
Vaccination
During each model cycle of ∼7 hours (100 per 30 days), a proportion of subjects who reach predefined ages is vaccinated; subjects successfully vaccinated move from the state ‘susceptible’ (S) to ‘susceptible seroprotected’ (SR) or from ‘infected’ to ‘infected seroprotected’ (O to OR or M to MR, depending on whether the meningococcal strain is VP). To account for <100% vaccine efficacy, the proportion of subjects transitioning from the non-vaccinated to vaccinated state is efficacy-adjusted so that all subjects in the vaccinated state are both vaccinated and protected from disease.
Vaccine impact
An assumption of the model is that successful vaccination (seroconversion) confers complete protection against VP meningococcal disease (direct effect) and may confer some protection against VP carriage acquisition (indirect effect). Only those subjects carrying VP strains (i.e. ‘M’ compartment) are at risk of VP meningococcal disease, while carriers of non-VP strains (‘O’ compartment) are at risk of non-VP disease.
Importantly, the model does not allow for direct transitions from carriage with VP meningococci to carriage with non-VP meningococci, or vice versa. As with previously published MenC vaccination models [Reference De Wals and Bouckaert18], it is assumed that co-infection with non-VP and VP meningococci does not occur; therefore, carriers of N. meningitidis of a VP strain are protected from infection with non-VP strains. Moreover, consistent with MenC models [Reference De Wals and Bouckaert18], transmission was dynamically modelled only in VP meningococci, and force of infection was assumed constant for non-VP meningococci.
No vaccine cross-protection for non-VP meningococcal strains was assumed, so successfully vaccinated individuals acquired infection with non-VP meningococci at the same rate as non-vaccine protected individuals.
Infection
During each model cycle, susceptible subjects (SR, S) may become infected and acquire carriage of a VP meningococcal strain (M) or a non-VP meningococcal strain (O); they then transition to the infected compartment corresponding to their infection and vaccination status. Most carriers of VP or non-VP meningococci recover without developing invasive meningococcal disease (IMD) and, in time, return to the susceptible state. A small proportion develop VP disease (‘M’ to ‘MD’) or non-VP disease (‘O’ or ‘OR’ to ‘OD’). Subjects who recover from meningococcal disease return to the non-vaccinated susceptible state.
The detailed differential model equations are available in the Supplementary online material.
Ageing
All model compartments are repeated for each 1-year age stratum; thus, for each health state there are 100 compartments (ages 0–99 years). At yearly intervals, all surviving subjects move to the next age stratum. A maximum life expectancy of 100 years was assumed so all subjects not dying before this age (of meningococcal disease or other conditions) exit the model at the end of their 99th year. Each year, a new birth cohort of subjects aged 0 years is introduced into the ‘non-vaccinated susceptible’ (S) compartment.
Model parameters
Model population
The model was populated with UK-specific historical and projected demographic data (2010 data) [13, 19, 20]. The age distribution of the UK population is incorporated into the model in 1-year age groups. The population is assumed to change each year due to ageing, births and deaths. As birth projections were only available until 2033, births were assumed constant thereafter.
Epidemiology/number of VP cases
Meningococcal cases
Data for 2001–2010 from governmental sources for England and Wales [21, 22], Scotland (A. Smith-Palmer, written communication April 2011) and Northern Ireland [23] were used to determine the age-stratified average annual number of meningococcal cases. Lacking data for Northern Ireland, the age distribution of serogroup B and C cases for 2001–2006 was assumed to match that of 2007–2010. The age distribution of serogroup W-135 and other cases was assumed to match the 2007–2010 age distribution of all laboratory-confirmed cases. Cases defined as ‘other’ or ‘non-groupable’ were redistributed by serogroup based on the distribution of groupable cases. To reflect the reduced number of cases since the MenC vaccination began, only data from 2006 to 2010 were averaged for serogroup C.
As evidence suggests that the officially reported numbers underestimate the true burden of disease [Reference Campbell24, Reference Trotter25], calculation of cases for the base-case scenario used Health Protection Agency (HPA) data (for IMD) corrected to align with hospital admissions data (hospital episode statistics; HES) for meningococcal meningitis, meningococcaemia and other meningococcal infections. This correction factor for underreporting for England was calculated as the percentage difference between cases reported to the HPA for England and Wales compared to population weighted cases reported to the HES for England only from 2000/2001 to 2010/2011 [26]. For Northern Ireland, the factor was calculated as the percentage difference in the total (confirmed and probable) and confirmed cases reported to the Public Health Agency for Northern Ireland from 2000 to 2010; no correction has been applied for Scotland.
VP meningococcal cases
These are defined as cases of serotypes B, C, W-135 and Y disease for which Bexsero provides effective coverage. This is consistent with the methods used for evaluating the MenC vaccine when introduced into the UK vaccination programme in 1999. The mathematical models used to evaluate conjugated MenC vaccines, proven to elicit bactericidal antibodies leading to killing of serogroup C meningococci in vitro, considered only ‘relevant’ meningococcal cases, i.e. those cases caused by meningococci effectively targeted by a MenC vaccine. Cases caused by meningococci of other serogroups could not be affected by the new MenC vaccines and therefore were not considered in those evaluations.
Preliminary evidence suggests that Bexsero has the potential to kill meningococci of multiple serogroups due to the vaccine antigens shared across serogroups [Reference Claus15, Reference Hong16]. To estimate the coverage potential of Bexsero against circulating meningococcal strains, a meningococcal antigen-typing system (MATS) was developed. This method is based on (and therefore correlates with) killing of strains in the serum bactericidal assay using human complement (hSBA) from pooled infant sera.
Based on MATS strain coverage, an estimated 73% of serogroup B cases, 79% of serogroup C cases, 82% of serogroup W-135 cases and 21% of serogroup Y cases are expected to be VP [Reference Donnelly27]. Consistent with the methods used for evaluation of MenC vaccines, only those ‘relevant’ cases were considered in the present evaluation. A correlation was established between MATS relative potency and killing of serogroup B strains in the hSBA by pooled immune serum from people who have been vaccinated [Reference Donnelly27]; however, the correlation of MATS and hSBA has not been established for other N. meningitidis serogroups. Consequently, the MATS estimation of coverage in non-serogroup B (serogroups C, W-135, Y) is based on bactericidal thresholds derived from relating MATS relative potency levels to killing of serogroup B strains by infant serum pools.
Model calibration
The model was calibrated to ensure that it accurately simulates the incidence of meningococcal carriage and disease in the UK population. A published WAIFW (who acquires infection from whom) matrix (table S8.4. b in [Reference Mossong28]) was used to compute transmission rates from the age-stratified force-of-infection data.
The age-specific distribution of meningococcal carriage was obtained from a meta-analysis of carriage estimates in multiple countries [Reference Christensen6]. As carriage values have been published for 0–74 years only, values were carried forward to 75–99 years assuming the same decline (0·1% per year) as reported for years ⩾72. The meta-analysis reported overall carriage only; therefore, it was assumed that the distribution of VP carriage in each age group mirrors the distribution of VP cases.
Distribution of case-fatality rates across age groups was obtained from [Reference Shigematsu29]; adjustment factors can be applied to modify the absolute case fatalities depending on the modelled scenario.
Similar to previously published methods [Reference Trotter, Gay and Edmunds30], the model was calibrated in three steps: (1) the age-specific force of infection (λ) was calibrated for the starting population and the age-specific carriage target values; (2) the probabilities of effective contact (β) were solved for each susceptible and carrier age group combination; and (3) the age-specific risk of disease given carriage (θ) was calibrated to UK cases reported over the last 10 years [21]. The same methods and pre-defined classes of functions as reported in the appendix of [Reference Trotter, Gay and Edmunds30] have been used to model the force of infection as well as the risk of disease given infection. Maximum-likelihood methods have then been applied to fit the force-of-infection parameters and the risk of disease given infection parameters to the carriage and disease data.
Time-frame
In the base-case analysis, meningococcal transmission and outcomes (cases) were tracked over a 5-year and a 100-year time-frame.
Model inputs
The movement between compartments in the model (Fig. 1) is dependent on the values of the input parameters and the model equations. The base-case parameter values with corresponding data sources are shown in Table 1. Rates of movement between compartments are assumed to follow an exponential distribution in which a constant rate is applied to the population in the relevant compartment.
Table 1. Model input parameters

HPV, Human papillomavirus; n.a., not applicable.
Vaccination strategies evaluated
Mathematical modelling allows optimization of the age at which vaccination strategies are implemented, as well as targeting the catch-up programme to the age cohorts yielding the most value. Four basic vaccination strategies consistent with the product label have been evaluated. In order to assess the impact of catch-up programmes on the case reduction within the first years after implementation of a vaccine programme, the early infant vaccination has been tested in combination with a 5-year and a 17-year catch-up, respectively. Finally, an adolescent component has been added to the combination programmes in addition; all strategies evaluated are summarized in Table 2. All vaccination programmes mentioned were compared to the current UK standard of care of two MenC doses at ages 3 and 4 months, and the combined Hib and MenC conjugate vaccine given as a booster between ages 12 and 13 months.
Table 2. Components of vaccination strategies assessed

It is assumed that Bexsero will be added to the current MenC vaccination programme and hence all benefits are assumed to be incremental to the current vaccination schedule.
Uncertainty analysis
For the combination of routine vaccinations in early infants and adolescents, together with a 5-year catch-up programme (Table 2), a probabilistic sensitivity analysis was performed with respect to the number of cases prevented vs. the current standard of care. Parameters assessed included: likelihood of successful vaccination; vaccine persistence (waning antibodies); vaccine uptake; recovery from carriage; case fatality. Narrow ranges were used when parameter value accuracy was well established (e.g. vaccination uptake); wider ranges were used for parameters with less accuracy (e.g. waning rates) (Table 1). In addition, the number of calibrated cases was varied between 0·62 and 1·68 of the base-case values, reflecting the range of cases annually in the UK between 2001 and 2010, with the age distribution of cases assumed to be the same as in the base case. For the probabilistic sensitivity analysis, estimates were drawn from a uniform distribution for waning, recovery from carriage and vaccination uptake parameters, while estimates for all probabilities, including the probability of successful vaccination vs. VP strains were drawn from a beta distribution.
A random-number generator was used to ‘draw’ parameter values from each distribution. Next, the model was re-calibrated using the drawn values for carriage recovery rate and disease incidence. The model was then run to generate estimates of the percentage reduction of VP cases. This process of drawing and replacing parameters and then calibration and running the model was repeated 1000 times. Finally, the number of cases prevented standardized to the case distribution associated with today's standard of care was calculated for each model run; the 25th ranked and 975th ranked number of cases prevented determined the upper and lower bounds of the confidence intervals (CIs).
RESULTS
Model calibration
Results of the calibration of the age-specific risk of VP disease to the observed incidence of meningococcal disease are presented in Figure 2. The fact that cases of meningococcal disease are reported in 5-year cohorts in the UK leads to a suboptimal model fit in teenagers and young adolescents. The risk of disease given carriage was highest in infants (1/200) and declined steeply with age, reaching a plateau (1/20 000) in the population aged >40 years.

Fig. 2. Model calibration: observed and predicted cases of vaccine-preventable disease by age.
Model analysis
The estimated number of cases of meningococcal disease under the current standard of care (i.e. without Bexsero vaccination) varies over time because of the projected changes in the population structure. Under the current standard of care, the model predicts 1640 cases of meningococcal disease in the first year, 8048 within the first 5 years (Table 3) and an annual average of 1547 cases over the model's 100-year time horizon (Table 4). To allow for easier interpretation given population changes over time, projected long-term vaccination outcomes are reported relative to the current standard of care.
Table 3. Five-year cumulative number (per cent reduction compared to current standard of care) of vaccine-preventable meningococcal cases, by vaccination strategy and age

* Numbers do not add up exactly due to rounding error.
Table 4. One-hundred-year cumulative number (per cent reduction compared to current standard of care) of vaccine-preventable meningococcal cases, by vaccination strategy and age

* Numbers do not add up exactly due to rounding error.
First 5 years of vaccination programme
Model results confirm that the early infant vaccination strategy (Table 2) provides the best direct protection in infants (Table 3). The early infant vaccination strategy is estimated to prevent 2089 cases within the first 5 years of the vaccination programme, including a 55% reduction in VP cases in infants aged <1 year vs. the current standard of care. Under this strategy, >50% of VP cases in infants aged ⩽2 years are averted, whereas <2% of VP cases in teenagers and adults are averted through indirect protection (Table 3). Overall, the number of preventable cases is reduced by 26%.
In comparison with routine infant vaccination programmes, routine adolescent vaccination programmes are characterized by a slow onset of protection from meningococcal disease. In the first 5 years of a vaccination programme, routine adolescent vaccination would prevent 655 cases of meningococcal disease vs. the standard of care, i.e. 31% of the cases averted with early infant vaccination.
Catch-up programmes
Short-term catch-up programmes in the first year(s) of the vaccination programme promote a faster reduction of VP cases. The larger the catch-up programmes, the faster and more complete the protection from meningococcal disease (Table 3). Compared with an early infant programme alone, adding a 5-year/17-year catch-up prevents an additional 1389/3253 cases, respectively, within the first 5 years of the vaccination programme, leading to a 43%/66% reduction of VP cases in year 5 of the vaccination programme vs. the current standard of care (Table 3).
One-hundred-year model time horizon
Routine vaccination strategies in the first 2 years of life can lead to significant reductions in VP cases within 5 years of introduction of the vaccination programme but reach a ‘plateau’ (steady state) about 40 years after the start of the programme (Fig. 3). The addition of a catch-up component to such programmes accelerates reduction in cases; however, if the catch-up programme does not lead to the eradication of VP disease, the long-term incidence level will converge in the long-term to the same ‘plateau’ as the routine vaccination programme without a catch-up component (Fig. 3).

Fig. 3. Estimated percentage of vaccine-preventable meningococcal cases relative to current standard of care in the UK.
Adding adolescent vaccination
Age at adolescent vaccination has been varied from 11 to 16 years, showing that vaccination programmes at higher age lead to higher reduction of meningococcal cases when assuming constant vaccination rates. However, the differences between strategies were small. In practice, however, it is assumed that high rates of routine vaccination are more difficult to achieve in subjects aged ⩾15 years. If a routine infant programme at ages 2, 3, 4 and 12 months together with routine adolescent vaccination at 14 years is combined with a 5-year catch-up programme, results suggest that a 1-year catch-up in infants between 12 and 24 months should be administered, together with an adolescent catch-up covering ages 15–18 years (strategy G). In the first 5 years, strategy G is estimated to reduce the number of VP cases by 69% in children aged ⩽24 months, and by 48% in the overall population. Over the first 10 years of the vaccine programme, 75% of cases aged <24 months and 59% of expected cases would be prevented compared to the standard of care. Over a 100-year time-frame, strategy G would prevent 94% of cases (Table 4).
Impact on carriage acquisition
Indirect protection of the unvaccinated population will occur only if the vaccine reduces carriage acquisition. Assuming a 100-year time horizon, the vaccine's impact on carriage acquisition and disease prevention under strategy G is shown in Figure 4. Assuming ⩾55% vaccine efficacy vs. carriage acquisition, Bexsero will prevent ⩾90% of VP cases; with only an assumed 30% impact on carriage acquisition, Strategy G nevertheless is estimated to prevent almost 70% of such cases. Assuming no vaccine impact on carriage acquisition at all, Bexsero will prevent almost 40% of VP cases (Fig. 4).
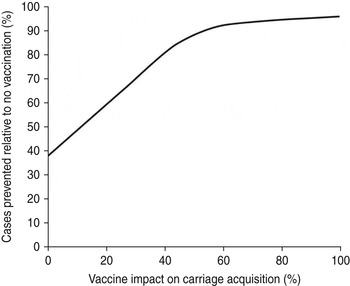
Fig. 4. Impact of carriage acquisition on the number of cases prevented by the base-case programme.
Uncertainty analysis
Results from the probabilistic sensitivity analysis of strategy G indicate that Bexsero is expected to prevent 93·5% (95% CI 84·9–96·5) of VP cases over 100 years. Figure 5 shows the percentage reduction in VP meningococcal disease cases over the modelled 100-year time-frame relative to the standard of care, with the 2·5% and 97·5% percentiles from the probabilistic sensitivity analysis.

Fig. 5. Estimated percentage of vaccine-preventable meningococcal cases for the base case, relative to no vaccination (current standard of care) shown with 2·5% and 97·5% percentiles from the probabilistic sensitivity analysis.
DISCUSSION
We developed a mathematical model to compare alternative vaccination programmes for the prevention of meningococcal disease in the UK setting, using similar methods and model structures as recently published [Reference Christensen34]. As recommended by international guidelines [35], we used a dynamic model to fully capture the clinical value of a meningococcal vaccine. In contrast, static models assume a fixed force of infection and do not capture potential herd protection; they may, therefore, greatly overestimate the remaining cases of disease after the introduction of vaccination and underestimate the potential benefits of a vaccination programme [13].
Results indicate that routine adolescent vaccination alone cannot achieve the high protection levels within the first 5 years of a vaccination programme seen with routine vaccination in early infants. This is explained by the relatively low number of meningococcal cases in adolescents and the associated low number of cases prevented as a result of direct protection, compared to infants. Moreover, the results confirm that the earlier infants are successfully vaccinated, the better their protection against meningococcal disease. Indeed, meningococcal disease can occur very early in life with a considerable number of cases reported in children aged <6 months. Vaccination programmes in which the first vaccine is given at 6 months or later cannot prevent these early infections.
Overall, catch-up programmes promote faster and greater reductions in meningococcal cases, but this trend will converge in the long term to the same ‘plateau’ as the respective routine vaccination programme without a catch-up component. Our results suggest that the elimination of VP cases requires a twofold approach: (1) a routine infant immunization programme to provide direct protection to those at greatest risk; (2) persistent reduction of the carriage reservoir, primarily in adolescents and young adults, through on-going vaccination. The reduction of preventable cases through indirect protection provided by routine adolescent vaccination can exceed that of direct protection and can lead to the elimination of VP meningococcal disease, assuming a sufficiently high rate of vaccination uptake. The results are sensitive to assumptions about vaccine impact on carriage acquisition. An OMV-based vaccine could be shown to reduce the likelihood of carriage by 85% during an on-going MenB outbreak in the Normandy region of France [Reference Delbos36]; OMV is one of the four components of Bexsero. Another study designed to assess pharyngeal carriage impact of the vaccine in a population with known high pharyngeal carriage rates supports the proof of concept that 4CMenB would provide herd protection against meningococcal disease but does not allow quantifying the reduction of individual carriage acquisition [Reference Read37]. However, an appropriate translation of the individual carriage impact into an estimate of herd protection can only be based upon results of further studies after the implementation of large-scale vaccination programmes. Based on the evidence and in lieu of any model to predict herd protection, the precedent from previous MenC vaccination programmes [Reference Ramsay8] of 67% has been chosen for 4CMenB.
There are inherent limitations in disease modelling and this was no different for our evaluation. In particular, there were limited data on relevant parameters and assumptions were made about their likely value and the variability that may exist around them. Evidence-based data were used where available. For example, we used clinical trial-derived likelihood of successful vaccination, laboratory-evaluated strain coverage and expected vaccine uptake, although even for these values some uncertainty remains. As with many new vaccines, long-term clinical data are unavailable, e.g. vaccine persistence for Bexsero is currently not fully assessed. In such cases, expert opinion and previous experience were used to define appropriate parameter values and any possible variability around these values.
The reasons for the unpredictable ‘natural fluctuation’ of cases of meningococcal disease over time, the changes of serogroup distribution within a country over time as well as the differences across geographies are not yet fully understood. To reflect these phenomena the average of reported numbers from 2001 to 2010 has been used for the base case (adjusting for the decline in incidence due to MenC vaccination) whereas the full range of reported numbers has been considered in the sensitivity analyses. Since 2010, the numbers have declined further. Efforts should be taken to fully understand the underlying reasons for the observed fluctuation of IMD incidence.
We did not consider the seasonality of meningococcal disease. Seasonality needs to be considered if the intention is to estimate the expected reduction of VP cases in the short term (over the first few years) after the start of the vaccination programme. The suboptimal model fit between estimated and observed meningococcal disease numbers, particularly in school-aged children and teenagers (Fig. 2), is not considered as a major limitation of this study. As mentioned, the relative poor model fit is explained by the fact that reported disease cases are grouped into 5-year cohorts – and the incidence numbers obtained from the fitted model are believed to reflect the reality better than the grouped numbers reported. Fitting more complex functions may require more parameters, which are believed to offset any potential gains from obtaining a better model fit. Further investigations in this area may be needed.
Estimates of the proportion of circulating meningococcal disease preventable by the vaccine can be considered conservative, as the method used for MATS does not include potential synergistic effects between vaccine antigens and does not consider coverage from non-PorA P1·4 components (PorA P1·4 is the immunodominant protein antigen contained in the OMV derived from MenB strain NZ98/254) of the OMV. Moreover, vaccine coverage was estimated based on post-vaccination sera collected from infants aged 12 months; coverage is expected to be greater in older age groups such as adolescents and adults.
We did not incorporate strain replacement into the current model. Because Bexsero contains multiple components and half of strains tested are covered by more than one Bexsero antigen, it is likely to retain effectiveness if one antigen is down-regulated or mutated. Thus, based on the characteristics of Bexsero and epidemiological evidence from previous vaccination programmes with serogroup-specific meningococcal vaccines, we considered the theoretical risk to be low for replacement by serogroups or specific strains within a serogroup that were not targeted by the vaccine.
Finally, our intention was to identify a Bexsero vaccination schedule that achieves both rapid reduction of the overall number of VP meningococcal cases and long-term elimination of VP meningococcal disease. Based upon our findings, such a programme may comprise routine administration of Bexsero to infants as early as possible, e.g. at ages 2, 3, 4 and 12 months, routine adolescent vaccination with two doses of Bexsero administered at 14 years and an adolescent catch-up programme in the first year of the vaccination programme. The catch-up programme should at least cover vaccination of all toddlers between ages 12 and 24 months which, in the case of Bexsero, would require two doses, as well as vaccination of the four adolescent cohorts between ages 15 and 18 years, again with two doses of Bexsero. If implemented in the UK, such a programme would potentially prevent about 150 000 cases and 15 000 deaths over a 100-year period.
SUPPLEMENTARY MATERIAL
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S095026881300294X.
ACKNOWLEDGEMENTS
This research was funded by Novartis Vaccines and Diagnostics AG. Editorial assistance was provided by Dr Ian Wright of Anthemis Consulting Ltd, funded by Novartis Vaccines and Diagnostics AG. Technical assistance was provided by Ms. Morgan Kruse of OptumInsight as a paid consultant to Novartis Vaccines and Diagnostics AG.
DECLARATION OF INTEREST
Karen Clements, Lisa McGarry, Gregory Hill are current or former employees of OptumInsight and were employed as paid consultants to Novartis Vaccines and Diagnostics AG.













