INTRODUCTION
Salmonella is one of the most important causative agents of foodborne infection in both developed and developing countries [Reference Cardinale1]. In the USA, it has been estimated that about 87% of all Salmonella-confirmed cases are foodborne, with 10% due to person-to-person infection, and 3% to pets [Reference Buzby and Roberts2]. Poultry and poultry products are among the main food sources most often incriminated in outbreaks of human salmonellosis [Reference Capita3, Reference Tauxe4] and infection frequently occurs as a result of cross-contamination from equipment, utensils and workers' hands with subsequent handling of raw carcasses and products, in addition to the consumption of undercooked poultry meat [Reference Yildirim5].
In recent years, there has been an increase in the incidence of human salmonellosis that is more difficult to treat due to the appearance of multidrug-resistant strains, especially S. Typhimurium, which have been isolated from various foods of animal origin worldwide [Reference Threlfall6]. The high prevalence of antimicrobial-resistant bacteria throughout the food industry is probably due to widespread overuse of common antimicrobials as therapeutics, prophylactics or growth promoters in food animals [Reference Capita and Alonso-Calleja7].
Although all the serovars of S. enterica are considered potentially pathogenic, there are considerable differences in their virulence to humans and this has been attributed to the absence or presence of plasmids carrying virulence-associated genes [Reference Porwollik8]. The invasion A (invA) gene is unique to all Salmonella serovars and is an internationally recognized marker for the rapid detection of Salmonella genus [Reference Rahn9]. The invA gene is required for invasion of the organism into host cells [Reference Torpdahl10], while the enterotoxin (stn) gene encodes a protein which mediates severe diarrhoea has also been utilized as a PCR target for the detection of Salmonella strains [Reference Prager11]. The Salmonella plasmid virulence gene, spvC, is believed to increase the growth rate of salmonellae in host cells and affect their interaction with the host immune system [Reference Gulig12].
Due to the increased incidence of resistant or multi-resistant Salmonella isolates worldwide against many commonly used antimicrobials, this study set out to determine the prevalence, serotypes, presence of virulence genes, and antimicrobial resistance of Salmonella isolates from chicken carcasses and giblets from retail outlets in Mansoura city, Egypt.
MATERIALS AND METHODS
Sample collection and bacteriological analysis
In total, 200 chicken samples (50 each of whole chicken carcasses, drumsticks, gizzards and livers) were randomly collected from 25 retail shops and supermarkets, of different sanitation levels, distributed in Mansoura city, Egypt on 10 occasions during the period June–November 2012. Each of the 25 shops was visited twice for sampling. On each sampling occasion, five shops were visited, and four samples (whole chicken carcass, drumstick, gizzard and liver) were taken from each shop. Each sample was packaged individually into a sterile impermeable polyethylene bag, labelled and transferred within 1 h in an icebox at ~4°C to the food hygiene laboratory for bacteriological analyses. The test portion for analysis was 25 g outer skin of whole chicken carcasses, skin plus muscle of drumsticks, and tissue from gizzards or livers excised aseptically with a sterile scalpel for each individual sample.
Each test portion was transferred into a sterile homogenizer flask containing 225 ml of sterile buffered peptone water (Oxoid, UK) and homogenized for 1 min in a stomacher (Seward Medical, UK). The homogenate was incubated at 37°C for 24 h then 0·1 and 1 ml volumes were aseptically added to 10 ml each of Rappaport Vassilliadis (RV) broth (Oxoid) and Muller–Kauffmann tetrathionate/novobiocin (MKTTn) broth (Oxoid), and incubated at 42°C for 24 h and 37°C for 24 h, respectively. These broths were subcultured on xylose-lysine-desoxycholate (XLD) agar (Oxoid) and Brilliant Green agar (BGA) with sulfadiazine (Neogen Corp., USA) which were incubated at 37°C for 24 h, and at 35°C for 24 h, respectively. Up to five typical (pink colonies with or without black centres) or suspected colonies of presumptive Salmonella were subcultured onto nutrient agar slopes and incubated at 37°C for 24 h for further biochemical and serological identification.
Identification of presumptive Salmonella isolates was performed according to standard methods [13] and carbohydrate fermentation profile using the API Rapid 20E system (bioMérieux, France) according to the manufacturer's instructions. Biochemically confirmed Salmonella isolates were serotyped by slide agglutination with O and H polyvalent antisera (Wellcome Diagnostic, UK).
Molecular analysis
Genomic DNA was prepared by a method described previously [Reference Choo14]. Salmonella Typhimurium (RIMD 1 985 009) and Escherichia coli K12DH5α were used as positive and negative control strains, respectively, for the presence and absence of invA, stn and spvC genes. The primers for PCR amplification of invA (244 bp) were as described previously [Reference Chiu and Ou15]. For PCR amplification of stn, two oligonucleotide primers (forward: 5′-CTTAATCGCGCCGCCATGCTGTT-3′; reverse: 5′-CATGAACTGGCGCAGGTGAT-3′) were constructed to produce an amplified band size of 480 bp. For amplification of spvC, two primers (forward: 5′-AACGGTTCCTCACGTAAAGCCTGT-3′; reverse: 5′- ACCAAATGCGGAAGATGCCGGTAT-3′) produced an amplified band size of 580 bp. PCR was performed in a 15-μl volume comprising 1 μl Salmonella DNA template, 1·6 μl each of forward and reverse primers (3 pmol each), 3 μl dNTPs (2 mm), 7·5 μl of 2 × PCR buffer for KOD FX, and 0·3 μl KOD FX DNA polymerase (Toyobo Co. Ltd, Japan). After an initial denaturation at 94°C for 2 min, 35 cycles (98°C for 10 s, 58°C for 30 s, 68°C for 30 s) were performed followed by a final extension at 68°C for 7 min. Amplified genes were verified by DNA sequencing with the BigDye Terminator v. 3.1 Cycle Sequencing kit (Applied BioSystems, USA) according to the manufacturer's instructions on an ABI Prism 3100 automated sequencer (Applied Biosystems). Nucleotide sequence data were analysed with GENETYXMAC software, v. 12 (GENETYX Corp., Japan). Homology searches of the obtained sequences against the already published genes in the GenBank were performed using Standard Nucleotide BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi).
Antimicrobial susceptibility tests
The antimicrobial susceptibility of Salmonella isolates was determined by an agar disk diffusion standard method [16] on Mueller–Hinton agar (Oxoid). Antibiotic discs were obtained from Difco (USA) and bioMérieux at the following drug concentrations: erythromycin (15 μg), nalidixic acid (30 μg), penicillin (10 IU), amoxicillin (30 μg), oxytetracycline (30 μg), sulphamethoxazole (25 μg), ampicillin (10 μg), streptomycin (10 μg), neomycin (30 μg), chloramphenicol (30 μg), norfloxacin (10 μg), ciprofloxacin (5 μg), kanamycin (30 μg), and gentamicin (10 μg). Isolates were classified as susceptible, intermediate or resistant according to National Committee for Clinical Laboratory Standards criteria [16], with intermediate susceptibility counted as resistant. E. coli ATCC 25 922 was used as a reference strain for antibiotic disc control. The multiple antibiotic resistance (MAR) index for each resistance pattern was calculated from the number of resistances to antimicrobials/total number of antimicrobials tested.
RESULTS
Salmonella spp. were detected in 34% (68/200) of all chicken samples, distributed as 16% (8/50), 28% (14/50), 32% (16/50) and 60% (30/50) among whole chicken carcasses, drumsticks, livers, and gizzards, respectively. By PCR, all 166 isolates were positive for the invA and stn genes; while 25·3% (42/166) carried the spvC gene (Fig. 1) and were mainly S. Typhimurium and S. Enteritidis. The specific primers used in the present study exhibited good amplification efficiency for detection of the target genes and produced sufficient DNA for sequence analysis to confirm the identity of the amplified genes.

Fig. 1. Representative agarose gel electrophoresis for PCR products showing the proper molecular size of (a) 244 bp for the amplified invA gene, (b) 480 bp for the amplified enterotoxin (stn) gene, and (c) 580 bp for the amplified Salmonella plasmid virulence (spvC) gene in Salmonela isolates recovered from chicken meat and giblets. Chromosomal DNA from the Salmonella isolates (n = 166) was used as a template for PCR amplification using specific primer sets for invA, stn and spvC genes. Three microliters of the PCR product were separated by electrophoresis on 1·2% agarose gel and visualized under UV light. M, DNA marker (gene ladder 100 bp) used as a reference for fragment size; lane C–, E. coli K12 DH5α used as negative control strain; lane C+, Salmonella Typhimurium (RIMD 1985009) used as positive control strain; lanes 1–14, representative positive strains.
Seven different serovars were distinguished among the Salmonella isolates. S. Enteritidis (37·3%) was the most frequent, followed by S. Typhimurium (30·1%), S. Kentucky (10·8%), S. Muenster (8·4%), S. Virchow (4·8%), S. Anatum (4·8%), and S. Haifa (1·2%). Four (2·4%) isolates could not be serotyped.
The distribution of Salmonella serovars among the chicken samples is shown in Table 1. S. Enteritidis and S. Typhimurium were recovered from all chicken parts, but S. Kentucky was not found in whole chicken carcass samples. Similarly, S. Muenster and the non-typable isolates were present in only liver and gizzard samples. S. Virchow was present in only drumstick and gizzard samples, and S. Anatum and S. Haifa were present in gizzard samples alone.
Table 1. Distribution of Salmonella serovars (n = 166) among chicken samples

All Salmonella isolates were resistant to erythromycin, penicillin, and amoxicillin (Table 2), while high resistance rates (>90%) were found for nalidixic acid (98·8%), sulphamethoxazole (96·4%), oxytetracycline (95·2%), and ampicillin (91·6%). The lowest rates were recorded for ciprofloxacin (63·9%), kanamycin (41·0%), and gentamicin (21·7%). The analysis of resistance profiles and MAR indexes of isolates by serotype is shown in Table 3. The great majority (92·8%, 154/166) of isolates showed resistance to ⩾3 of the 14 antimicrobials tested. Two-thirds of isolates (63·2%, 105/166) had a MAR index above the average (0·582) and within these isolates 13 resistance profiles encompassing eight to all 14 antimicrobials were identified. Among S. Enteriditis, 50/62 (80·6%), and all of 50 S. Typhimurium isolates were resistant to ⩾9 antimicrobials.
Table 2. Percentages of antimicrobial susceptibility of Salmonella species isolated from chicken carcasses and products (n = 166 isolates)

Table 3. Antibiotic resistant profiles and multiple resistance index (MAR) of Salmonella isolates from chicken carcasses and products (n = 166 isolates)
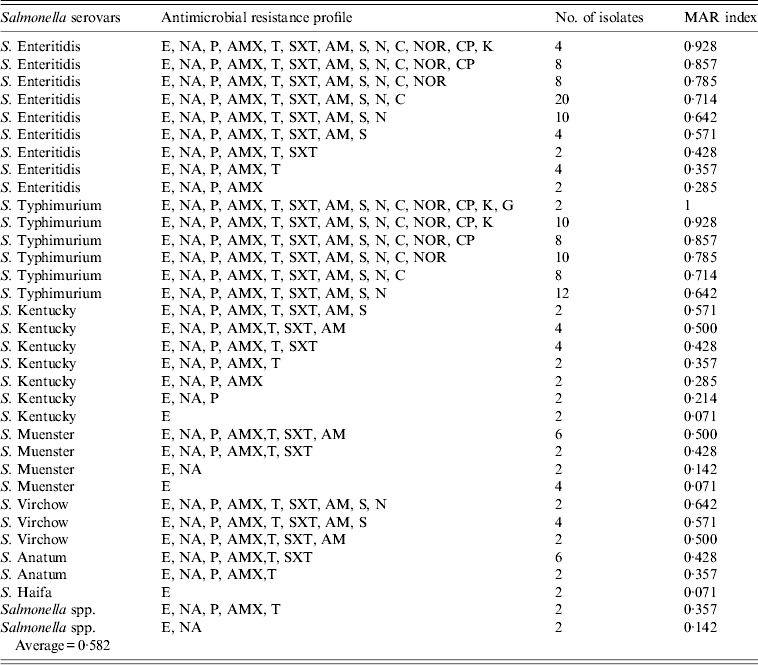
E (erythromycin, 15 μg); NA (nalidixic acid, 30 μg); P (penicillin, 10 IU); AMX (amoxicillin, 30 μg); T (oxytetracycline, 30 μg); SXT (sulphamethoxazole, 25 μg); AM (ampicillin, 10 μg); S (streptomycin,10 μg); N (neomycin,10 μg); C (chloramphenicol, 30 μg); NOR (norfloxacin, 10 μg); CP (ciprofloxacin, 5 μg); K (kanamycin, 30 μg); G (gentamicin, 10 μg).
Only 12 (7·2%) of the 166 isolates exhibited resistance to just one or two antimicrobials and these comprised the less frequently occurring serotypes, S. Haifa, S. Muenster and S. Kentucky.
DISCUSSION
Our results of the prevalence of Salmonella in whole chickens and drumsticks are in agreement with the prevalence rates of Salmonella in poultry meats recorded in different countries which range from 19·2% in fresh and frozen chicken carcasses in South Africa [Reference van Nierop17], 22% in Louisiana (USA) retail stores [Reference Lestari18], and 27% in retail market broiler chicken carcasses in Colombia [Reference Donado-Godoy19]. Higher Salmonella contamination rates have been reported from several studies ranging from 34% in Turkey [Reference Yildirim5] to 66% in Thailand [Reference Jaowapa20]. By contrast, only 0·6% of 168 samples of meat parts of broiler chickens tested in an earlier survey in Turkey [Reference Cetinkaya21] were contaminated with Salmonella, while all of the 127 poultry carcasses tested in Brazil were negative for these organisms [Reference de Freitas22]. Similarly for chicken giblets (gizzards, liver, heart) reported rates vary depending on the survey country, notably 86% in Thailand [Reference Jaowapa20], 34·5% and 41% of livers and gizzards, respectively, in Ethiopia [Reference Molla and Mesfin23] and 3% in Argentina [Reference Favier24].
The wide variation in Salmonella prevalence in chicken meat from different studies could be attributed to geographical differences, sampling techniques, bacteriological methods as well as slaughter hygiene and cross-contamination of products at different stages of chicken dressing and preparation. The observed greater contamination of gizzard and liver samples over other samples may reflect greater manipulation of these organs in addition to contamination from the crop and intestinal contents during evisceration. As a result of the control programme of Salmonella in chickens reared for meat production in the UK, the number of S. Enteritidis- and S. Typhimurium-infected breeding chicken flocks is currently very low owing to the introduction of strict control measures among which include management, cleaning and disinfection, hen vaccination, pest control, biosecurity, monitoring, and the potential use of other aids in the control of Salmonella [25]. Wider application of such programmes may therefore be beneficial in reducing contamination rates in some countries.
The invA gene has been widely used for the detection of Salmonella spp. in food samples and its presence is highly associated with other virulence genes such as the stn gene which contributes to the pathogenicity process, primarily diarrhoea [Reference Chopra26]. In S. Typhimurium and S. Enteriditis the virulence plasmid is known to increase the growth rate of the microorganisms at sites beyond the intestine and aid colonization of deeper tissue. Our finding of spv genes among these serovars is therefore consistent with early reports of their association with highly invasive serovars.
Gizzard samples proved to be the most contaminated (30/50) of tissue samples and yielded each of the seven serovars detected from all samples. The absence of certain serovars such as S. Virchow, S. Anatum, and S. Haifa from some of the other sample types no doubt reflects their low overall frequency and perhaps less cross-contamination during preparation.
The predominance of S. Enteriditis and S. Typhimurium in this survey echoes the results of several other surveys of foodborne salmonellosis in the literature, albeit with differences in counties in the rates of these serovars [Reference Foley27]. However, low rates of S. Enteritidis (5·9%) were notable from Turkey [Reference Tauxe4], and 1·3% (1/73) from Brazil [Reference de Freitas22]; similarly low frequencies of S. Typhimurium have been reported from other surveys [Reference van Nierop17, Reference Dogru28]. S. Kentucky accounted for 10·8% of all our isolates which contrasts markedly with 59·5% and 41% in studies from the USA and Ireland, respectively [Reference Parveen29, Reference Whyte30].
As expected, resistance to erythromycin, nalidixic acid and penicillin was almost universal and high resistance rates were evident for most of the antimicrobials tested which is consistent with the literature [Reference Yildirim5, Reference Dogru28, Reference Álvarez-Fernández31]. In the context of agents that would be considered for the treatment of diarrhoeal salmonellosis, it was surprising to find relatively poor levels of clear susceptibility to chloramphenicol (22%) norfloxacin (32·5%), and ciprofloxacin (36%). Although 78% of isolates were susceptible to gentamicin, this drug would generally be used parenterally and only for extraintestinal infections. The relatively lower rates of resistance to norfloxacin, ciprofloxacin, kanamycin and gentamicin could be attributed to their limited use in animal production. Our findings corroborate the widely held view that poultry is a major source of multidrug-resistant Salmonella, and underlines the value of antibiotic susceptibility surveys for selecting appropriate treatment options for salmonellosis caused by strains of poultry origin. The data also serve to highlight the need for implementation of antimicrobial stewardship programmes in developing countries, including Egypt, to optimize their use for treatment, and reduce the spread and development of antimicrobial-resistant strains.
In conclusion, we have demonstrated that a high proportion of chicken carcasses and giblets sold in Mansoura, Egypt were contaminated with Salmonella, predominantly S. Typhimurium and S. Enteritidis, the great majority of which were multidrug resistant. Hence, chicken meat and their products constitute a significant problem for public health and this calls for better antimicrobial stewardship to reduce the unnecessary use of antimicrobials in the food industry.
DECLARATION OF INTEREST
None.









