There are two predominant types of diets for feeding experimental animals: non-purified diet and purified diet. The primary ingredients of a non-purified diet are derived from natural sources, such as maize, wheat bran, defatted soyabean, defatted rice bran, fishmeal, defatted milk, soyabean oil and brewer's yeast. In contrast, a purified diet is made up of refined ingredients( Reference Reeves, Nielsen and Fahey 1 ). Not surprisingly, purified and non-purified diets have different impacts on the physiology and pathophysiology of certain diseases in animals. For instance, in our previous study, we had found that fructo-oligosaccharide supplementation exacerbated the symptoms of dextran sulphate sodium-induced colitis in mice fed a purified diet, but attenuated the symptoms in those fed a non-purified diet( Reference Goto, Takemura and Ogasawara 2 ). In addition, we had reported that Candida albicans, an opportunistic fungal pathogen, successfully colonises the gut of mice fed a purified diet but not that of mice fed a non-purified diet( Reference Yamaguchi, Sonoyama and Kikuchi 3 ). A purified diet is widely used for nutritional studies. However, a purified diet is quite different from the human diet, which contains a wide variety of ingredients. Therefore, studies using a purified diet may not properly reflect physiological and pathophysiological phenomena under certain circumstances. Nevertheless, the different impacts of feeding non-purified and purified diets on the physiology and pathophysiology of animals have not been studied intensively.
The gastrointestinal tract harbours various strains of microbes, predominantly anaerobic bacteria, which have symbiotic relationships with the development and health of host animals( Reference Cheesman and Guillemin 4 ). Lactobacilli have the ability to grow and colonise in the acidic conditions( Reference Tannock 5 ) of the stomach and are the predominant bacterial species in the stomach of laboratory rodents throughout their lifespan( Reference Dubos, Schaedler and Costello 6 – Reference Savage and Blumershine 9 ). The stomach of mice is compartmentalised into the forestomach, lined with keratinised stratified squamous epithelium, and the glandular stomach, lined with columnar secreting epithelium. Lactobacilli colonise the forestomach. Previous studies based on histological examination and conventional cultivation have demonstrated that the number of lactobacilli colonising the forestomach is much lower in mice fed a purified diet than in those fed a non-purified commercial rodent diet( Reference Yamaguchi, Sonoyama and Kikuchi 3 , Reference Brockett and Tannock 10 ). In addition, we estimated the number of lactobacilli in the gastric tissue by cultivation-independent molecular biological analyses based on microbial 16S rRNA gene sequences( Reference Sahasakul, Takemura and Sonoyama 11 ). Real-time quantitative PCR using Lactobacillus-specific primers has revealed that mice fed purified and non-purified diets have 3·27 (se 0·22) and 6·02 (se 0·12) log copies/g tissue of 16S rRNA gene, respectively( Reference Sahasakul, Takemura and Sonoyama 11 ). In addition, Lactobacillus gasseri and Lactobacillus johnsonii have been reported to be the predominant Lactobacillus species associated with the gastric tissue of mice fed a non-purified diet( Reference Sahasakul, Takemura and Sonoyama 11 ). However, the mechanism by which the diets influence Lactobacillus colonisation in the stomach remains to be elucidated.
A non-purified diet contains many types of non-digestible ingredients including soluble viscous fibre, whereas a purified diet is made up of refined ingredients and contains cellulose as the sole source of non-digestible ingredients( Reference Reeves, Nielsen and Fahey 1 ). Because supplementation of soluble viscous fibre slows the gastric emptying (GE) rate of a solid meal( Reference Brown, Worlding and Rumsey 12 – Reference Tadesse 17 ), mice fed a non-purified diet may have a lower GE rate than those fed a purified diet. Clearly, longer retention time of a solid meal in the stomach allows sufficient time for bacteria to grow( Reference Stevens and Hume 18 ). Therefore, we postulated that a lower GE rate in mice fed a non-purified diet allows lactobacilli to colonise in the stomach. In addition, ghrelin, a peptide hormone that is produced in the stomach and promotes GE( Reference Chen, Asakawa and Fujimiya 19 ), may be responsible for the different GE rates in mice fed non-purified and purified diets. We tested these hypotheses in the present study.
Materials and methods
Animals and diets
All study protocols were pre-approved by the Animal Use Committee of the Hokkaido University (approval no. 08-0139), and all mice were maintained in accordance with the Guidelines for the Care and Use of Laboratory Animals of the Hokkaido University.
Female specific pathogen-free BALB/cCr Slc mice (age 5 weeks) were purchased from Japan SLC, Inc. and housed in standard plastic cages in a temperature-controlled (23 ± 2°C) room under a 12 h light–12 h dark cycle. They were allowed free access to food and water. After acclimatisation with a non-purified commercial rodent diet (MR Stock; Nihon Nosan Kogyo; Table 1) for 1 week, the mice were fed the non-purified diet, a purified diet prepared according to the composition of AIN-93G( Reference Reeves, Nielsen and Fahey 1 ), or the purified diet supplemented with sugarbeet fibre (SBF, a gift from Nippon Beet Sugar Manufacturing Company Limited) or carboxymethyl cellulose (CMC, Na salt, high viscosity; MP Biomedicals; Table 2). SBF (200 g/kg diet) was added to the purified diet as a substitute for cellulose and α-maize starch. CMC (40 g/kg diet) was added to the purified diet as a substitute for α-maize starch. According to Aritsuka et al. ( Reference Aritsuka, Tanaka and Kiriyama 20 ), the composition of SBF (g/100 g) is as follows: moisture, 4·5; total dietary fibre, 81·1 (cellulose, 23·0; hemicellulose, 22·0; pectin, 19·0; lignin, 3·0; and unidentified matter, 14·4); protein (N × 6·25), 9·0; lipid, 0·6; sucrose, 1·5; ash, 3·0. We detected no culturable lactobacilli in all the experimental diets (data not shown).
Table 1 Composition of the non-purified diet*
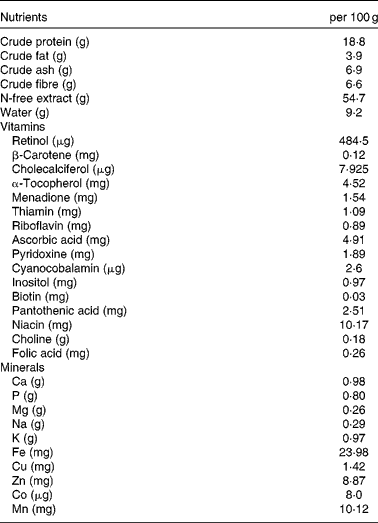
* The non-purified diet (MR Stock) was purchased from Nihon Nosan Kogyo. Details regarding the nutrient content are available at http://lt.nosan.co.jp/ahg/list01.html (in Japanese).
Table 2 Composition of the purified diet
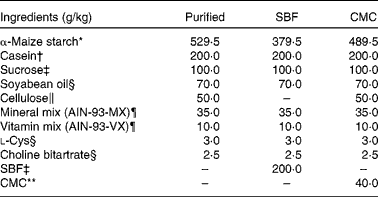
SBF, sugarbeet fibre; CMC, carboxymethyl cellulose.
* Purchased from Chuo-Shokuryou (Amylalpha CL).
† Purchased from New Zealand Dairy Board (ALACID).
‡ Gifted from Nippon Beet Sugar Manufacturing Company Limited.
§ Purchased from Wako Pure Chemical Industries Limited.
∥ Purchased from Advantec Toyo Limited (cellulose powder type D).
¶ Mineral mixture and vitamin mixture are identical to AIN-93-MX and AIN-93-VX, as reported by Reeves et al. ( Reference Reeves, Nielsen and Fahey 1 ), respectively. These were purchased from Nihon Nosan Kogyo Company.
** Purchased from MP Biomedicals.
Experimental design
In Expt 1, the mice were randomly allocated to two groups (n 6 per group) and fed either the non-purified or the purified diet. GE was measured according to the method of Asakawa et al. ( Reference Asakawa, Inui and Ueno 21 ) 2 weeks after feeding the mice with the test diets. In brief, after 16 h of food deprivation, the mice were fed ad libitum with 0·2 g of their respective diets for 1 h, and food intake was then measured by weighing the uneaten diet. The mice were anaesthetised by diethyl ether inhalation and killed by exsanguination from the carotid artery. Following a laparotomy, the stomach was excised and cut open along the greater curvature, and contents were then collected and lyophilised. GE was calculated according to the following formula:
 $$\begin{eqnarray} GE\ (\%) = (1 - (dry\,\,weight\,\,of\,\,the\,\,food\,\,recovered\,\,from\,the\,\,stomach/weight\,\,of\,\,\,the\,\,food\,\,intake))\times 100. \end{eqnarray}$$
$$\begin{eqnarray} GE\ (\%) = (1 - (dry\,\,weight\,\,of\,\,the\,\,food\,\,recovered\,\,from\,the\,\,stomach/weight\,\,of\,\,\,the\,\,food\,\,intake))\times 100. \end{eqnarray}$$
Gastric tissue samples were washed gently with cold sterile PBS to remove the remaining contents and used for the enumeration of lactobacilli as described below.
In Expt 2, the mice were randomly allocated to two groups (n 12 per group) and fed either the non-purified diet or the purified diet. Mice in each group were further divided into two subgroups (n 6 per group) and given either tap water or ampicillin (1 mg/ml)-supplemented tap water as drinking-water. Similar to Expt 1, GE was measured in the mice 2 weeks after feeding the test diets. EDTA plasma was separated from blood samples and used for measuring ghrelin concentrations as described below. A portion of the gastric tissue samples was used for the enumeration of gastric tissue-associated lactobacilli similar to Expt 1. The remainder of the tissue samples was immediately frozen in liquid N2 and stored at − 80°C for the isolation of RNA.
In Expt 3, the mice were randomly allocated to four groups (n 6 per group) and fed the non-purified diet, the purified diet or the purified diet supplemented with SBF or CMC. Similar to Expt 1, GE was measured in the mice 2 weeks after feeding the test diets. Gastric tissue samples were used for the enumeration of gastric tissue-associated lactobacilli similar to Expt 1.
Enumeration of lactobacilli
Gastric tissue samples were added to 1 ml of anaerobic phosphate buffer and, after shaking vigorously for 1 min, the washings were collected. The washings were centrifuged at 700 g for 1 min, and the supernatant was then collected. This washing procedure was repeated once again, and the supernatants were combined. The samples were diluted stepwise 10-fold with anaerobic phosphate buffer, and a sample (0·05 ml) of each dilution was inoculated onto Lactobacillus selection agar( Reference Mitsuoka, Sega and Yamamoto 22 ). Anaerobic incubation was carried out at 37°C for 48 h using the AnaeroPack system (Mitsubishi gas). After incubation, the number of colonies was counted and expressed as logarithmic colony-forming units.
Measurement of serum ghrelin concentrations
The concentrations of des-acyl and acylated ghrelin in EDTA plasma samples with 1 mm-p-hydroxymercuribenzoic acid were measured using commercial ELISA kits (rat des-acyl ghrelin enzyme immunoassay kit and rat acylated ghrelin enzyme immunoassay kit, respectively; Bertin Pharma) according to the manufacturer's instructions.
Analysis of ghrelin mRNA expression
Total RNA was isolated from tissue homogenates using the TRIzol reagent (Invitrogen) according to the manufacturer's instructions. After digestion of genomic DNA with RQ1 RNase-Free DNase (Promega), approximately 10 ng of total RNA were annealed with the Oligo(dT) 12–18 primer (Invitrogen) at 70°C for 10 min, and first-strand complementary DNA was then synthesised using M-MLV Reverse Transcriptase (Invitrogen), followed by RNA digestion with DNase-Free RNase H (Invitrogen). Real-time quantitative PCR was carried out using Thermal Cycler Dice TP800 (Takara). Primer sequences for ghrelin and ghrelin O-acyltransferase (GOAT) were identical to those described by Gahete et al. ( Reference Gahete, Córdoba-Chacón and Salvatori 23 ). Primer sequences for 18S rRNA were identical to those described by Johnson et al. ( Reference Johnson, Leitner and Rivest 24 ). Amplification was carried out in a 25 μl reaction volume containing 12·5 μl of 1 × SYBR Premix Ex Taq (Takara), 200 nmol/l of each primer and 1 μl of template complementary DNA. The reaction was carried out under the following conditions: 95°C for 10 s, followed by forty cycles at 95°C for 15 s and 60°C for 30 s, with dissociation curve analysis at 95°C for 15 s, 60°C for 30 s and 95°C for 15 s. The relative gene expression levels of each sample were normalised to those of 18S rRNA.
Statistical analyses
Results are presented as means with their standard errors. To compare mean values, Student's t test or two-way ANOVA with Tukey–Kramer post hoc test was used. Correlations between the parameters were assessed using Pearson's correlation test. GraphPad Prism for Macintosh (version 5.0; GraphPad Software, Inc.) was used for the analyses. P values < 0·05 were considered to be statistically significant.
Results
Gastric emptying and Lactobacillus number in mice fed the non-purified and purified diets (Expt 1)
Conventional cultivation analyses revealed that mice fed the non-purified diet had a significantly higher number of gastric tissue-associated lactobacilli than those fed the purified diet (Fig. 1(A)), which was consistent with the results of our previous study( Reference Yamaguchi, Sonoyama and Kikuchi 3 ). GE was significantly higher in mice fed the purified diet than in those fed the non-purified diet (Fig. 1(B)). There was a significant negative correlation between GE and the number of gastric tissue-associated lactobacilli in mice fed the non-purified and purified diets (Fig. 1(C)).
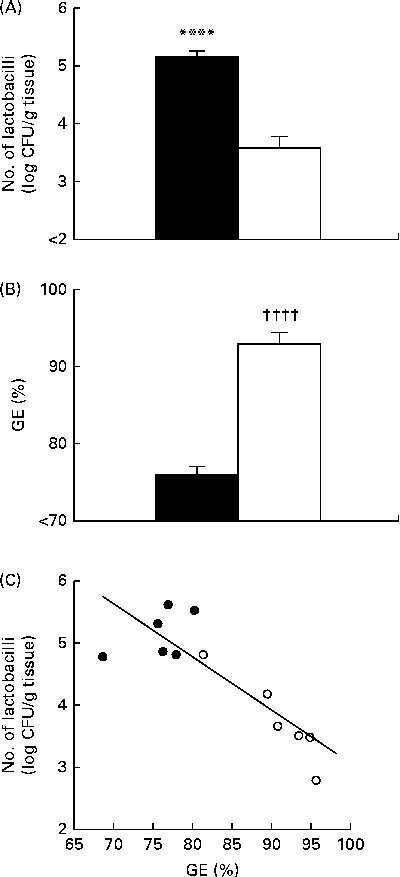
Fig. 1 Gastric Lactobacillus number and gastric emptying (GE) in BALB/cCr Slc mice fed the non-purified and purified diets for 2 weeks (Expt 1). (A) The number of gastric tissue-associated lactobacilli estimated by cultivation analyses. (B) GE estimated by measuring the food recovered from the stomach. Values are means (n 6), with their standard errors represented by vertical bars. **** Mean value was significantly different from that of the purified diet-fed group (P< 0·0007; Student's t test). †††† Mean value was significantly different from that of the non-purified diet-fed group (P< 0·0001; Student's t test). ■, Non-purified diet; □, purified diet. (C) The relationship between GE and the number of lactobacilli, assessed by Pearson's correlation test (r= 0·8730; P= 0·0002). ●, Non-purified diet-fed mice; ○, purified diet-fed mice. CFU, colony-forming units.
Gastric emptying and Lactobacillus number in mice fed the non-purified and purified diets and administered with ampicillin (Expt 2)
In line with Expt 1, in mice not subjected to ampicillin administration, the number of gastric tissue-associated lactobacilli was significantly higher in mice fed the non-purified diet than in those fed the purified diet (Fig. 2(A)). In mice subjected to ampicillin administration through the drinking-water, the number of gastric tissue-associated lactobacilli was less than 102 colony-forming units/g tissue in both mice fed the non-purified diet and those fed the purified diet (Fig. 2(A)). Thus, ampicillin administration successfully eliminated lactobacilli in the stomach. GE was significantly influenced by the diets, but not by ampicillin administration (Fig. 2(B)). Thus, GE remained higher in mice fed the purified diet than in those fed the non-purified diet in both those subjected and not subjected to ampicillin administration, and ampicillin administration had no impact on GE. In mice not subjected to ampicillin administration, there was a significant negative correlation between GE and the number of gastric tissue-associated lactobacilli (Fig. 2(C)).
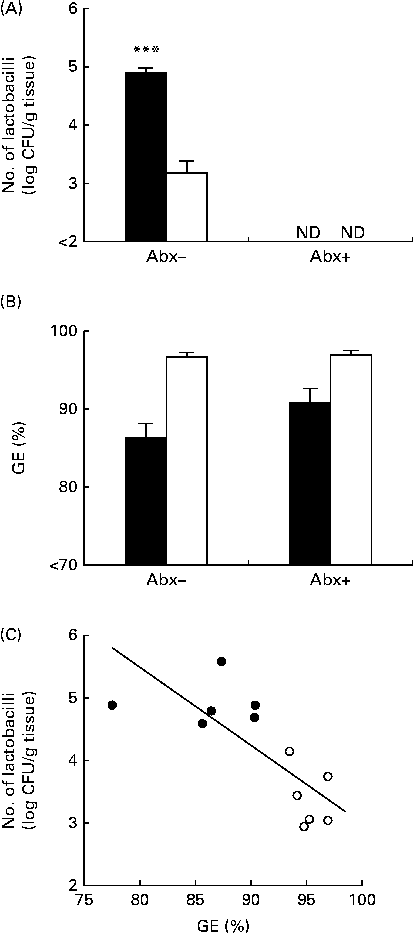
Fig. 2 Gastric Lactobacillus number and gastric emptying (GE) in BALB/cCr Slc mice fed the non-purified and purified diets with and without ampicillin administration (Abx+ and Abx − , respectively) for 2 weeks (Expt 2). (A) The number of gastric tissue-associated lactobacilli estimated by cultivation analyses. Values are means (n 6), with their standard errors represented by vertical bars. **** Mean value was significantly different from that of the purified diet-fed group (P< 0·0001; Student's t test). ND, not detectable. (B) GE estimated by measuring the food recovered from the stomach. Values are means (n 6), with their standard errors represented by vertical bars. For GE, there was a significant diet effect (P< 0·0001; two-way ANOVA). No effects were detected for Abx (P= 0·1098; two-way ANOVA) and the diet × Abx interaction (P= 0·1556; two-way ANOVA). ■, Non-purified diet; □, purified diet. (C) The relationship between GE and the number of lactobacilli in mice not subjected to ampicillin administration, assessed by Pearson's correlation test (r= 0·7995; P= 0·0018). ●, Non-purified diet-fed mice; ○, purified diet-fed mice. CFU, colony-forming units.
Ghrelin levels in mice fed the non-purified and purified diets and administered with ampicillin (Expt 2)
Both diets and ampicillin administration had no influence on the expression of gastric ghrelin mRNA of the mice (Fig. 3(A)). Similarly, there were no significant differences in the plasma concentrations of des-acyl ghrelin among the groups (Fig. 3(B)). However, the plasma concentrations of acylated ghrelin, an active form of ghrelin, were higher in mice fed the purified diet than in those fed the non-purified diet, whereas ampicillin administration had no effects (Fig. 3(C)). Accordingly, GE exhibited a positive correlation with the plasma concentrations of acylated ghrelin (Fig. 3(D)). There were no significant differences in the mRNA levels of GOAT, the enzyme mediating ghrelin acylation, among the groups (Fig. 3(E)).

Fig. 3 Ghrelin levels in BALB/cCr Slc mice fed the non-purified and purified diets with and without ampicillin administration (Abx+ and Abx − , respectively) for 2 weeks (Expt 2). (A) The relative levels of ghrelin mRNA in gastric tissue, estimated by real-time quantitative PCR (RT-qPCR). (B, C) The plasma concentrations of des-acyl and acylated ghrelin, respectively. (D) The relationship between plasma acylated ghrelin concentrations and gastric emptying (GE), assessed by Pearson's correlation test (r= 0·3943; P= 0·0511). ●, Non-purified diet-fed mice; ○, purified diet-fed mice. (E) The relative levels of ghrelin O-acyl transferase (GOAT) mRNA in gastric tissue, estimated by RT-qPCR. (A, E) The values of ghrelin and GOAT mRNA were normalised to the value of 18S rRNA, and the values are expressed relative to the average values in mice fed the non-purified diet without ampicillin administration, which is set to 1·0. Values are means (n 6), with their standard errors represented by vertical bars. For ghrelin mRNA, des-acyl ghrelin and GOAT mRNA, there were no significant diet (P= 0·4342, P= 0·5468 and P= 0·3326), Abx (P= 0·0699, P= 0·7595 and P= 0·0669) and diet × Abx interaction (P= 0·3839, P= 0·4229 and P= 0·1937) effects (two-way ANOVA), respectively. For acylated ghrelin, there was a significant diet effect (P= 0·0578), but no effects were detected for Abx (P= 0·6578) and the diet × Abx interaction (P= 0·9938) (two-way ANOVA). ■, Non-purified diet; □, purified diet.
Gastric emptying and Lactobacillus number in mice fed the non-purified diet, the purified diet, and the purified diet supplemented with sugarbeet fibre or carboxymethyl cellulose (Expt 3)
The number of gastric tissue-associated lactobacilli was significantly higher in mice fed the non-purified diet than in those fed the purified diet and the purified diet supplemented with SBF or CMC (Fig. 4(A)). There were no significant differences in the number of lactobacilli between mice fed the purified diet and those fed the purified diet supplemented with SBF or CMC. GE was significantly higher in mice fed the purified diet and the purified diet supplemented with CMC than in those fed the non-purified diet (Fig. 4(B)). Mice fed the purified diet exhibited significantly higher GE rates than those fed the purified diet supplemented with SBF or CMC. There were no significant differences in GE between mice fed the purified diet supplemented with SBF and those fed the non-purified diet and between mice fed the purified diet supplemented with SBF and those fed the purified diet supplemented with CMC. There was a significant negative correlation between GE and the number of gastric tissue-associated lactobacilli among all the groups (Fig. 4(C)).

Fig. 4 Gastric Lactobacillus number and gastric emptying (GE) in BALB/cCr Slc mice fed the non-purified diet, the purified diet, and the purified diet supplemented with sugarbeet fibre (SBF) or carboxymethyl cellulose (CMC) for 2 weeks (Expt 3). (A) The number of gastric tissue-associated lactobacilli estimated by cultivation analyses. (B) GE estimated by measuring the food recovered from the stomach. Values are means (n 6), with their standard errors represented by vertical bars. a,b,cMean values with unlike letters were significantly different (P <0·05; Tukey–Kramer test followed by two-way ANOVA). ■, Non-purified diet; □, purified diet; ![]() , SBF;
, SBF; ![]() , CMC. (C) The relationship between GE and the number of lactobacilli in mice, assessed by Pearson's correlation test (r= 0·7601; P< 0·0001). ■, Non-purified diet-fed mice; □, purified diet-fed mice; ■, SBF-supplemented purified diet-fed mice; □, CMC-supplemented purified diet-fed mice. CFU, colony-forming units.
, CMC. (C) The relationship between GE and the number of lactobacilli in mice, assessed by Pearson's correlation test (r= 0·7601; P< 0·0001). ■, Non-purified diet-fed mice; □, purified diet-fed mice; ■, SBF-supplemented purified diet-fed mice; □, CMC-supplemented purified diet-fed mice. CFU, colony-forming units.
Discussion
The present study clearly demonstrated that the colonisation levels of lactobacilli in the stomach are negatively correlated with GE in mice fed non-purified and purified diets. Because the diet form, i.e. liquid, powder or pellet, generally tends to influence GE, in the present study, the powder form of purified and non-purified diets was used. To evaluate whether GE is the cause or consequence of Lactobacillus colonisation in the stomach, mice were administered ampicillin in the drinking-water. Even when lactobacilli were eliminated by ampicillin administration, mice fed the purified diet had higher GE rates than those fed the non-purified diet, suggesting that higher GE rates are the cause, but not the consequence, of lower colonisation levels of lactobacilli in mice fed the purified diet. Thus, it appears likely that a purified diet accelerates GE, which prevents lactobacilli to persist and colonise in the stomach.
To test whether retardation of GE allows lactobacilli to colonise in the stomach, in the present study, mice were fed a purified diet supplemented with SBF or CMC, because supplementation of soluble viscous fibre reportedly retards GE of a solid meal( Reference Brown, Worlding and Rumsey 12 – Reference Tadesse 17 ). SBF is a naturally occurring fibre containing both insoluble fibre and soluble viscous fibre( Reference Aritsuka, Tanaka and Kiriyama 20 ), and CMC is a non-fermentable, viscous fibre that increases the viscosity of luminal contents. The Prosky fibre value of the non-purified diet used in the present study was 16 %( Reference Goto, Takemura and Ogasawara 2 ). Because SBF contains approximately 80 % of total dietary fibre( Reference Aritsuka, Tanaka and Kiriyama 20 ), we supplemented SBF at a concentration of 200 g/kg diet so that the total fibre concentration in the diet was 16 %. However, it was difficult to add CMC at a final concentration of 16 % because of the problem of evoking osmotic diarrhoea. According to Bär et al. ( Reference Bär, Til and Timonen 25 ) and our preliminary experiment (Y Sahasakul and K Sonoyama, unpublished results), we supplemented CMC at a concentration of 40 g/kg diet as a maximum non-observed adverse effect concentration. Mice fed the purified diet supplemented with SBF or CMC had lower GE rates than those fed the purified diet. These results are in agreement with previous observations in human volunteers supplemented with CMC( Reference Tadesse 17 ) and in pigs supplemented with sugarbeet pulp( Reference Guerin, Ramonet and LeCloarec 15 ). Although no significant differences in gastric Lactobacillus colonisation levels were observed between mice fed the purified diet and those fed the purified diet supplemented with CMC or SBF, there was a significant negative correlation between GE and Lactobacillus colonisation levels in mice fed the non-purified diet, the purified diet, and the purified diet supplemented with CMC or SBF. A non-purified diet contains many types of non-digestible ingredients including soluble viscous fibre, whereas a purified diet contains cellulose as the sole source of non-digestible ingredients( Reference Reeves, Nielsen and Fahey 1 ). In this context, we assumed that soluble viscous fibre in a non-purified diet might be responsible for the retardation of GE, which allows sufficient time for lactobacilli to grow in the stomach( Reference Stevens and Hume 18 ).
Among the gastrointestinal hormones, including motilin, cholecystokinin, glucagon-like peptide 1 and peptide YY, which are involved in the regulation of GE, only ghrelin is the hormone that is produced in the stomach and promotes GE( Reference Chen, Asakawa and Fujimiya 19 , Reference Ogiso, Asakawa and Amitani 26 ). Therefore, the present study focused on ghrelin as a possible mediator responsible for the different GE rates in mice fed non-purified and purified diets. Ghrelin is a motilin-related peptide that was discovered in the stomach and acts as an endogenous ligand of growth hormone secretagogue receptor( Reference Kojima, Hosoda and Date 27 ). Ghrelin gene products are present in various forms including acylated ghrelin, des-acyl ghrelin, des-Gln14-ghrelin and obestatin( Reference Ogiso, Asakawa and Amitani 26 ). Acylated ghrelin, a 28-amino acid peptide, is produced by a unique post-translational modification of O-n-octanoylation at serine 3, while des-acyl ghrelin, lacking O-n-octanoylation, is also produced in the stomach and remains the major molecular form secreted into the circulation( Reference Chen, Asakawa and Fujimiya 19 ). In the present study, we observed no difference in gastric ghrelin mRNA levels and plasma des-acyl ghrelin concentrations between mice fed the non-purified diet and those fed the purified diet. However, mice fed the purified diet had higher plasma concentrations of acylated ghrelin than those fed the non-purified diet, which is consistent with a previous report that consumption of carob pulp rich in insoluble fibre reduces postprandial acylated ghrelin concentrations, but fails to affect des-acyl ghrelin concentrations in humans( Reference Gruendel, Garcia and Otto 28 ). Therefore, it appears likely that acylated ghrelin is responsible for the different GE rates in mice fed the non-purified and purified diets. The enzyme GOAT mediates O-n-octanoylation of ghrelin( Reference Gutierrez, Solenberg and Perkins 29 , Reference Yang, Brown and Liang 30 ). Assuming that the activity of GOAT is regulated at the pre-translational levels, the present study compared the gastric mRNA levels of GOAT of mice fed a non-purified diet and those of mice fed a purified diet. However, we observed no significant influence of diets on the mRNA levels. Thus, further studies are required to clarify the mechanisms by which dietary fibre affects the plasma concentrations of acylated ghrelin.
It should be denied that Lactobacillus colonisation in the stomach affected the plasma concentrations of acylated ghrelin in mice fed the non-purified diet, because the elimination of lactobacilli by ampicillin administration had no effects on acylated ghrelin concentrations. Interestingly, chronic Helicobacter pylori infection in mouse stomach has reportedly been shown to cause increased mononuclear cell infiltration into the gastric corpus and larger gastric area, delay GE, and elevate plasma acylated ghrelin and cholecystokinin concentrations( Reference Bercik, Verdú and Foster 31 ). In the present study, we observed no infiltration of inflammatory cells into the gastric tissues of mice fed the non-purified diet (data not shown), suggesting symbiotic colonisation, but not infection, by lactobacilli. Accordingly, there is a possibility that opportunistic infection by gastric lactobacilli, probably occurring by suboptimal immune defence, affects the plasma concentrations of acylated ghrelin.
Lactobacillus species generally demonstrate increased sensitivity at pH values < 3, although differences exist between species and specific strains( Reference Hood and Zottola 32 , Reference Jin, Ho and Abdullah 33 ). Therefore, one may assume that the pH value of gastric contents might be responsible for the different levels of Lactobacillus colonisation in mice fed the purified and non-purified diets. According to our previous observation( Reference Yamaguchi, Sonoyama and Kikuchi 3 ), however, the pH values of gastric contents in mice fed the purified and non-purified diets were 4·0 and 4·3, respectively. Thus, the possibility that the pH of gastric contents is associated with the growth of bacteria should be excluded.
Alternatively, dietary constituents may directly influence the growth of lactobacilli in the stomach, because we had previously observed that the number of lactobacilli in stomach contents, as in the case of the gastric tissue, was higher in mice fed a non-purified diet than in those fed a purified diet( Reference Yamaguchi, Sonoyama and Kikuchi 3 ). For instance, the fat content and composition may be responsible for the different GE rates and/or Lactobacillus colonisation levels in mice fed the purified and non-purified diets. Dietary fat is well known to influence GE, and the fat content is indeed different between the purified and non-purified diets (7·0 and 3·9 %, respectively). In addition, Brockett & Tannock( Reference Brockett and Tannock 10 ) reported that the relative amounts of palmitic and oleic acids in a laboratory-prepared basic diet are correlated with the number of tissue-associated lactobacilli in the stomach of mice. Additionally, they reported that the commercially prepared pelleted food might contain substances that modify the toxic effects of fatty acids on lactobacilli. In our preliminary in vitro experiments, however, the growth of L. johnsonii isolated from the stomach of mouse was not found to differ between cultures supplemented with the purified and non-purified diets (unpublished results). Thus, further studies are required to determine whether dietary constituents directly influence the growth of lactobacilli in the stomach.
It is well known that the stomach of laboratory rodents is colonised by lactobacilli( Reference Dubos, Schaedler and Costello 6 – Reference Savage and Blumershine 9 ). A purified diet, which is widely used for nutritional studies, has been shown to reduce the number of lactobacilli colonised in the stomach( Reference Yamaguchi, Sonoyama and Kikuchi 3 , Reference Brockett and Tannock 10 ). The present study shed light on the mechanism for the reduction of Lactobacillus colonisation levels in mice fed a purified diet. Although it remains to be elucidated whether the different colonisation levels of lactobacilli in the stomach influence the physiology of the host animals, we should keep in mind that a purified diet is quite different from the human diet and that studies using a purified diet may not properly reflect physiological and pathophysiological phenomena under certain circumstances.
Acknowledgements
The present study was inspired by Dr Kazunari Ushida of Kyoto Prefectural University.
The authors' contributions are as follows: Y. S. and K. S. designed the research and wrote the manuscript; Y. S. and N. T. conducted the research. All authors read and approved the final manuscript.
None of the authors has any conflicts of interest to declare.










