Similar to the rest of sub-Saharan Africa, South Africa is plagued by the burden of chronic infectious diseases including tuberculosis and HIV( 1 ) as well as a rising burden of non-communicable diseases( Reference Young, Critchley and Johnstone 2 ). Recently, studies have shown that vitamin D deficiency or insufficiency is associated with an increased risk of CVD( Reference Giovannucci, Liu and Hollis 3 ), diabetes( Reference Pittas, Lau and Hu 4 ), cancers( Reference Lin, Manson and Lee 5 , Reference Schottker, Haug and Schomburg 6 ), infections( Reference Martineau, Nhamoyebonde and Oni 7 ) and autoimmune conditions( Reference Pludowski, Holick and Pilz 8 ) in addition to its classical effects on bone.
Although its definition remains a concern, several studies on vitamin D deficiency across the world suggest that it is a global health problem( Reference Wahl, Cooper and Ebeling 9 , Reference Mithal, Wahl and Bonjour 10 ). South African studies have been limited to only children( Reference Poopedi, Norris and Pettifor 11 ) or women( Reference Kruger, Kruger and Wentzel-Viljoen 12 ) or hospitalised or ill individuals( Reference Martineau, Nhamoyebonde and Oni 7 ), and the reported prevalence of vitamin D deficiency ranges from 8·0 % in African children to 62·7 % in adults with tuberculosis and/or HIV when using a cut-off value < 50 nmol/l to define deficiency.
To understand the health risks associated with vitamin D deficiency, it is important have knowledge of the determinants of 25-hydroxyvitamin D (25(OH)D) concentrations. The objective of the present study was to determine the vitamin D status of two ethnic groups in Johannesburg and to identify the main determinants of 25(OH)D concentrations in each ethnic group, including body fat mass and body fat distribution. Factors such as sun exposure, age, fat mass, sex, ethnicity and lifestyle may influence the serum concentrations of this vitamin( Reference Mithal, Wahl and Bonjour 10 , Reference McGill, Stewart and Lithander 13 – Reference Sulistyoningrum, Green and Lear 15 ) and may be important contributors to vitamin D status. The serum concentration of 25(OH)D has been reported to be inversely associated with BMI and with subcutaneous adiposity (SCAT) as well as visceral adiposity (VAT)( Reference Cheng, Massaro and Fox 16 , Reference Ross, Manson and Abrams 17 ). We determined the contributions of 25(OH)D3 and 25(OH)D2 to total 25(OH)D concentrations using HPLC as several commonly used immunoassays preferentially detect 25(OH)D3 ( Reference Hollis 18 ).
Methods
Subjects
This was a cross-sectional study of ambulatory African and Asian-Indian adults living in the Greater Johannesburg Metropolitan area. Subjects were recruited via carers of the Birth to Twenty cohort( Reference Richter, Norris and De Wet 19 ). Carers were randomly selected from this study cohort and then asked whether they had family members interested in participating in the study. Exclusion criteria were pregnancy, breast-feeding and age below 18 years or above 65 years, impaired renal function as determined by self-reports, and mixed ancestry or Caucasian race. A total of 730 participants were recruited, and prevalence results are reported for 714 subjects (sixteen subjects were excluded either because they did not meet the inclusion criteria or because they had incomplete data). The sample size was calculated to ensure a statistical power of 90 % and a probability of less than 0·05 for detecting a 15 % inter-group difference in 25(OH)D concentrations. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki. The Human Ethics Committee of the University of the Witwatersrand approved all the procedures involving the subjects. Written informed consent was obtained from each subject. The study was conducted from July 2011 to April 2012.
Biochemical analysis
Venous blood samples were collected in EDTA tubes for the measurement of 25(OH)D concentrations after an overnight fast. Plasma 25(OH)D was run on a HPLC system (HPLC Shimadzu LC-10 ADVP; Shimadzu Corporation) with a photodiode array detector using the ClinRep® HPLC Complete Kit for 25(OH)D2/D3 in plasma and serum (Recipe)( Reference Janssen, Wielders and Bekker 20 ). The separated 25(OH)D2 and 25(OH)D3 were detected at a wavelength of 264 nm and chromatograms integrated using peak height. The sum of 25(OH)D2 and 25(OH)D3 was taken as total 25(OH)D. This analysis was carried out in a laboratory that participates in the Vitamin D External Quality Assessment Scheme (DEQAS) and achieved the performance targets set by the DEQAS. The intra-assay and inter-assay CV for 25(OH)D3 for controls at a mean of 61·7 nmol/l ranged from 0·36 to 9·4 % and those for controls at a mean of 222 nmol/l ranged from 2·1 to 5·5 %. For 25(OH)D2, the CV for the low control (mean 49·2 nmol/l) ranged from 6·8 to 9·7 % and that for the high control (mean 199 nmol/l) ranged from 1·1 to 5·7 %. The limit of quantification was 6·5 nmol/l for 25(OH)D3 and 11·0 nmol/l for 25(OH)D2. The level of 25(OH)D2 was below the limit of quantification in 9·2 % of the subjects and these cases were assigned a nominal value of 0. The following values were used to define vitamin D status: 25(OH)D concentrations < 30 nmol/l, vitamin D deficiency; 25(OH)D concentrations = 30–49·9 nmol/l, vitamin D insufficiency; 25(OH)D concentrations >50 nmol/l, vitamin D sufficiency( Reference Ross, Manson and Abrams 17 ).
Serum creatinine, serum Ca, serum phosphate and alkaline phosphatase concentrations were measured on the ADVIA 1800 (Siemens). The concentrations of parathyroid hormone (PTH) were measured using a chemiluminescent assay on a Centaur autoanalyser (Siemens). The intra-assay CV for PTH was 5·2 % at 4·3 pmol/l and 3·4 % at 23·7 pmol/l. The reference range in the study laboratory is 1·20–8·85 pmol/l.
Anthropometric measurements
Height (in cm) was measured using a wall-mounted stadiometer (Holtain Limited) and weight (in kg) was measured using a digital scale (Dismed, Inc.). Both instruments were calibrated weekly. The participants wore minimal clothing when being weighed. BMI was calculated as the participant's weight (kg) divided by his or her height squared (m2).
Abdominal VAT and SCAT were determined by ultrasound using a LOGIC ultrasound system (GE Healthcare) with a 2–5 MHz 3C-RS curved array transducer( Reference De Lucia Rolfe, Norris and Sleigh 21 ). Ultrasound visceral adipose tissue thickness was defined as the distance (cm) from the peritoneum to the vertebral bodies, and ultrasound subcutaneous adipose tissue thickness was defined as the depth (cm) from the skin to the linea alba. The scan depth was set at 15 cm for the visceral fat measurement and at 9 cm for the subcutaneous fat measurement to visualise the relevant anatomical structures. Both measurements were obtained at the site where the xiphoid line and waist circumference met. A single trained operator took all the measurements.
Total body adipose tissue was quantified by dual-energy X-ray absorptiometry (Hologic, software version 12.5:7; scan region 195 × 65 cm2 and weight limit 120 kg) according to standard procedures, with a CV of 0·7 % for fat-free tissue mass and that of 1·67 % for fat mass.
Sun exposure
Sun exposure was determined using a questionnaire, which assessed the participants' recollection of daily sun exposure over the previous week( Reference Hanwell, Vieth and Cole 22 , Reference Lee, Park and Kim 23 ). There were three options for time spent outdoors every day (0 = ≤ 5 min, 1 = 5–30 min and 2 = >30 min). There were four options for skin exposure while outdoors (1 = face and hands only; 2 = face, hands, and arms; 3 = face, hands, and legs; and 4 = bathing suit). A daily sun exposure score (min = 0; max = 8) was calculated using the product of the amount of time spent outdoors and the amount of skin exposed. Sun exposure scores obtained for the 7 d were then summed to obtain the weekly sun exposure score (min = 0; max = 56). Seasons of the year, for the statistical analysis, were categorised as winter (June to August), spring (September to November), summer (December to February) and autumn (March to May). This depended on when the study participants were assessed. The average daily sunshine hours for the study period were as follows: 9·4 h, winter; 9·8 h, spring; 7·8 h, summer; 8·4 h, autumn (Communication from the South African Weather Service 26th February 2013).
Dietary calcium and vitamin D intake
A trained worker administered a 7 d FFQ to each participant. Portion sizes were reported in household measures and then converted to weights using standard tables( Reference Langehoven, Conradie and Wolmarans 24 ). Food intake (g) was calculated for 7 d to be able to include quantities of less than 1 g/d in the analysis. Thereafter, the nutrient content was calculated and expressed as the average amount of nutrients consumed per d for each participant. For the purpose of the present study, Ca intake was assessed from the intake of dairy products, while vitamin D intake was determined from the intake of only eggs, margarine, butter and fish. In South Africa, there is no mandatory fortification of foods with vitamin D; however, most margarines are vitamin D fortified( Reference Poopedi, Norris and Pettifor 11 ). Vitamin D and Ca supplement use was assessed and recorded as ‘yes’ or ‘no’.
Smoking status, level of education and HIV status
Current smoking status (yes/no) was assessed during the interview. The level of education attained by the participants was categorised as follows: 0 – did not complete high school and 1 – completed high school and/or attained a post-high school qualification. The prevalence of HIV was quantified by the self-reporting of HIV status by each participant.
Statistical analysis
Statistical analysis was conducted using the Stata software package version 12 (StataCorp). The Shapiro–Wilk test was used to test for normality. Non-normally distributed data are reported as medians and interquartile ranges (IQR) and normally distributed data as means and standard deviations. Non-normally distributed data were transformed to achieve normality (log transformed or square root) before regression analysis. The Mann–Whitney U test was used to compare differences between the two ethnic groups for non-normally distributed data, the Student t test for normally distributed data and the χ2 test for categorical data. A P value < 0·05 was considered statistically significant. We compared differences in 25(OH)D concentrations between the two ethnic groups using ANOVA. Pairwise comparisons were used only if the overall ANOVA results were statistically significant. Bonferroni corrections were used for multiple comparisons.
The main aim of the present study was to identify the principal determinants of 25(OH)D concentrations in each ethnic group and therefore the data of the African and Asian-Indian subjects were analysed separately. Regression analysis was restricted to total 25(OH)D rather than to 25(OH)D2 and 25(OH)D3 individually. We carried out univariate Spearman's analysis to assess the strength of the relationship of 25(OH)D concentrations with parameters that are thought to influence 25(OH)D status. The following were considered as potential predictors based on previous evidence: age( Reference MacLaughlin and Holick 25 ); season( Reference Pettifor, Ross and Solomon 26 ); body fat( Reference Bostick, Kushi and Wu 27 ); sun exposure( Reference Langehoven, Conradie and Wolmarans 24 ); vitamin D or Ca supplementation( Reference Millen, Wactawski-Wende and Pettinger 28 ); renal function; smoking status( Reference Cutillas-Marco, Fuertes-Prosper and Grant 29 ); dietary intake of Ca and vitamin D( Reference Zgaga, Theodoratou and Farrington 30 ); PTH concentrations. These as well as anthropometric measures of subcutaneous, visceral and whole-body fat were used as independent variables in separate multivariate models for the African and Asian-Indian subjects in which 25(OH)D was the dependent variable. For each ethnic group, the season during which the lowest 25(OH)D concentrations were recorded was coded as 1 and the remaining seasons were grouped together and coded as 2. Winter was coded as 1 for the African subjects and spring for the Asian-Indian subjects. A backward stepwise multiple regression analysis was carried out until only variables with a P value < 0·05 remained. We adjusted all the models for sex and height. Due to collinearity, BMI and total body fat were not included in the same model. We tested for the collinearity of models that included VAT, SCAT and total body fat and found no collinearity as evidenced by a variance inflation factor < 10·0. We also tested for an interaction between ethnicity and body composition variables and found no significant interaction. To facilitate direct comparisons of the strengths of the associations, the results of the regression models are reported as standardised β-values.
Results
Demographic variables
Complete data were available for 714 subjects (371 Africans and 343 Asian-Indians). The demographic and biochemical characteristics of the participants are given in Table 1.
Table 1 Descriptive characteristics of the study participants† (Mean values and standard deviations (parametric data); medians and inter-quartile ranges (IQR; non-parametric data) and percentages)

eGFR, estimated glomerular filtration rate; 25(OH)D, 25-hydroxyvitamin D; Pi, inorganic phosphate; PTH, parathyroid hormone.
*** Median values were significantly different from that of males of the same ethnic group (P< 0·0005).
† In the present study, 25(OH)D deficiency was defined as 25(OH)D concentrations < 30 nmol/l and insufficiency as 25(OH)D concentrations = 30·0–49·9 nmol/l.
‡ For African females, n 191; for Asian-Indian females, n 180; for African males, n 180; and for Asian-Indian males, n 163.
The mean ages of the African and Asian-Indian subjects were similar (41·6 (sd 13·1) years (range 19–65) and 43·5 (sd 13·0) years (range 18–65; P= 0·06), respectively). More Asian-Indian subjects than African subjects had completed high school (P< 0·0001), and there were more smokers in the African group than in the Asian-Indian group (34 v. 17 %, P< 0·0001). None of the participants was using anti-epileptic medication, but 12·9 % of the Asian-Indian subjects and 1·4 % of the African subjects were on statin therapy. HIV status was determined by self-report: 87·7 % of the Black Africans reported their status as negative, 8·5 % reported being HIV positive and 3·7 % reported to have had never been tested; 94·5 % of the Asian-Indians reported their status as negative and 5·5 % reported to have had never been tested. To our knowledge, none of the participants suffered from metabolic conditions likely to affect vitamin D metabolism.
Anthropometry
Median BMI was in the overweight range for both groups. The Asian-Indian subjects had greater waist circumference, greater total body fat and greater body fat percentage than the African subjects (P< 0·0001 for all variables). VAT was similar between the two ethnic groups, but the Asian-Indian subjects had greater SCAT than the African subjects (Table 1).
Vitamin D status
Using the Shapiro–Wilk test, 25(OH)D was found to not be normally distributed. The African subjects had significantly higher 25(OH)D and 25(OH)D3 concentrations than the Asian-Indian subjects (see Table 1). Sex differences were observed in 25(OH)D as well as 25(OH)D3 concentrations. The prevalence of vitamin D deficiency (25(OH)D concentrations < 30 nmol/l) was 28·6 % in the Asian-Indian subjects compared with just over 5 % in the African subjects (Table 1) and was highest in the Asian-Indian women (37·9 % in Asian-Indian females and 17·9 % in Asian-Indian males, P< 0·0001; 6·7 % in African females and 2·8 % in African males, P= 0·08).
In both ethnic groups, 94·5 (sd 17·9) % of all the 25(OH)D measured was 25(OH)D3. Mean serum Ca and phosphate concentrations were normal and similar between the two groups. Plasma PTH concentrations were above the laboratory reference range in 12 % of the African subjects and 14 % of the Asian-Indian subjects.
Seasonal differences
As expected, seasonal differences were observed in 25(OH)D concentrations in both groups. Plasma 25(OH)D concentrations were 40–60 % higher in subjects from whom blood samples were collected in autumn than in those from whom blood samples were collected in winter/spring (Fig. 1). This variation was due to changes in 25(OH)D3 concentrations, with all the participants exhibiting maximal 25(OH)D3 concentrations in autumn. As expected, no significant seasonal difference was observed in 25(OH)D2 concentrations. The prevalence of 25(OH)D deficiency ( < 30 nmol/l) per season was as follows: 8 % in winter; 23 % in spring; 13 % in summer; < 1 % in autumn. The prevalence of 25(OH)D insufficiency ( < 50 nmol/l) per season was as follows: 40 % in winter; 31 % in spring; 30 % in summer; 4 % in autumn.
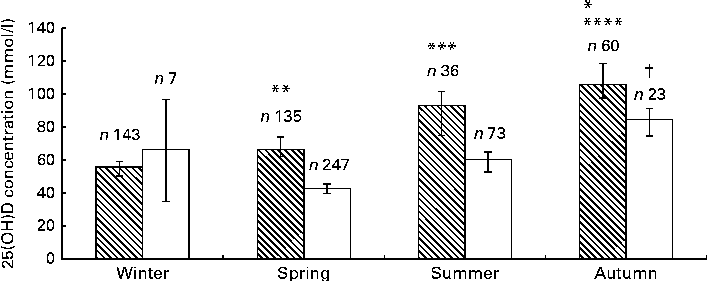
Fig. 1 25-Hydroxyvitamin D (25(OH)D) concentration across four seasons in Africans (![]() ) and Asian Indians (□). Values are means, with 95% confidence intervals represented by vertical bars. Results were adjusted for sex using ANCOVA. * In Africans, mean value was significantly different from that for summer (P< 0·05). ** In Africans, mean value was significantly different from that for winter (P= 0·005). *** In Africans, mean value was significantly different from that for winter (P= 0·001). **** In Africans, mean value was significantly different from that for winter and spring (P< 0·001). † In Asian Indians, mean value was significantly different from that for spring and summer (P< 0·001).
) and Asian Indians (□). Values are means, with 95% confidence intervals represented by vertical bars. Results were adjusted for sex using ANCOVA. * In Africans, mean value was significantly different from that for summer (P< 0·05). ** In Africans, mean value was significantly different from that for winter (P= 0·005). *** In Africans, mean value was significantly different from that for winter (P= 0·001). **** In Africans, mean value was significantly different from that for winter and spring (P< 0·001). † In Asian Indians, mean value was significantly different from that for spring and summer (P< 0·001).
Sun exposure scores were not significantly different between the ethnic groups but were different by sex: African males – 18·80 (sd 8·49) and African females – 16·73 (sd 11·33) (P= 0·02); Asian-Indian males – 18·71 (sd 10·19) and Asian-Indian females – 14·43 (sd 8·46) (P= 0·0001).
Supplementation and medication: Asian-Indian population
In the Asian-Indian population, 22 % of the subjects were on Ca supplementation and 15 % were on vitamin D supplementation (Table 1). The median concentrations of 25(OH)D were higher in Asian-Indian subjects on vitamin D supplementation (52·2 (IQR 32·7–73·2) v. 39·2 (IQR 26·9–55·1) nmol/l; P< 0·001) or on Ca supplementation (54·2 (IQR 35·8–80·4) v. 37·5 (IQR 26·3–53·8) nmol/l; P< 0·0001). The median concentrations of 25(OH)D in Asian-Indian males on statin therapy (49·3 (IQR 30·3–60·9) nmol/l) were not significantly different from those in males not on statin therapy (45·1 (IQR 34·0–60·9) nmol/l; P= 0·7), but were higher that those in Asian-Indian females on statin therapy (52·2 (IQR 30·1–80·8) nmol/l) compared with those in females not on statin therapy (34·4 (IQR 22·4–52·9) nmol/l; P= 0·04).
Supplementation and medication: African population
In the African population, 3 % of the subjects were on Ca supplementation and 2 % on vitamin D supplementation (P< 0·0001 for both comparisons with the Asian-Indian population) (Table 1).
Dietary intake of calcium and vitamin D
The median dietary Ca intake was significantly lower in the African subjects (223 (IQR 98·6–427) mg/d) than in the Asian-Indian subjects (336 (IQR 204–487) mg/d) (P< 0·0001). The median dietary vitamin D intake was higher in the African subjects (2·95 (IQR 1·55–5·12) μg/d) than in the Asian-Indian subjects (1·17 (IQR 0·50–2·11) μg/d) (P< 0·0001). Dietary vitamin D intake was ≥ 10 μg/d in 7·5 % of the African subjects and 1·2 % of the Asian-Indian subjects (P= 0·008). Similarly, dietary Ca intake was ≥ 1000 mg/d in 4·5 % of the African subjects and ≥ 1000 mg/d in 2·6 % of the Asian-Indian subjects (P= 0·002).
Univariate analysis for associations with 25-hydroxyvitamin D concentrations
The correlations of 25(OH)D concentrations with other variables are summarised in Table 2. Univariate analysis revealed that serum PTH concentrations were negatively correlated with 25(OH)D concentrations in both ethnic groups and weekly sun exposure score was significantly positively associated with 25(OH)D concentrations in the African subjects but not in the Asian-Indian subjects. In terms of body composition, VAT and SCAT as well as total body fat were negatively associated with 25(OH)D concentrations in the African subjects. In the Asian-Indian subjects, none of the body composition variables was significantly associated with 25(OH)D concentrations. There was no significant association of 25(OH)D status with age and dietary Ca or vitamin D intake in either group.
Table 2 Spearman's correlations of 25-hydroxyvitamin D (25(OH)D) concentrations with other variables (β Coefficients)
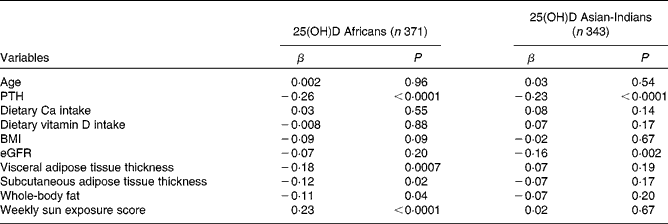
PTH, parathyroid hormone; eGFR, estimated glomerular filtration rate.
Predictors of 25-hydroxyvitamin D concentrations
To assess the predictors of 25(OH)D concentrations in more detail, we carried out multivariate analyses (see Table 3). In the African subjects (see model 1), season of blood sample collection, PTH concentrations and weekly sun exposure score explained 16 % of the variance in 25(OH)D concentrations. Season of blood sample collection and weekly sun exposure score were positive predictors of 25(OH)D concentrations, while PTH concentrations were negatively related to 25(OH)D concentrations.
Table 3 Multivariate regression models for 25-hydroxyvitamin D (25(OH)D) (Standardised β-coefficients)

PTH, parathyroid hormone.
* Independent variables used are age, season of blood sample collection, smoking status, Ca and vitamin D supplementation, sex, estimated glomerular filtration rate, weekly sun exposure, PTH concentrations, and subcutaneous, visceral and whole-body fat. The following were log transformed: PTH and 25(OH)D. Season: winter was the reference season for the African subjects and spring was the reference season for the Asian-Indian subjects (see the statistical analysis section for a full description of the coding). Female sex was coded as 0 and male sex as 1.
† R 2 are unadjusted values.
Season of blood sample collection, PTH concentrations, sex and Ca supplement use explained 17 % of the variance in 25(OH)D concentrations in the Asian-Indian subjects (see model 2, Table 3). Sex, season of blood sample collection and Ca supplement use were all positive predictors of 25(OH)D concentrations.
Discussion
To our knowledge, this is the first study to assess the determinants of 25(OH)D concentrations in African and Asian-Indian individuals living in Africa. As reported by Prentice et al. ( Reference Prentice, Schoenmakers and Jones 31 ), Africa is a heterogeneous continent with differences in latitude, climate, food availability, skin pigmentation and cultural practices. A systematic review of global vitamin D status by Wahl et al. ( Reference Wahl, Cooper and Ebeling 9 ) has reported that there is a lack of data from many African countries. Mean concentrations ranging between 25 and 49 nmol/l have been reported by a single South African study( Reference Charlton, Labadarios and Lombard 32 ), with higher concentrations being reported for West and Central Africa( Reference Wahl, Cooper and Ebeling 9 ). Hilger et al. ( Reference Hilger, Friedel and Herr 33 ) reported similar findings in a more recent systematic review of vitamin D status in populations worldwide. We have shown that the prevalence of 25(OH)D deficiency (28·6 %) is high in Asian-Indians living in Johannesburg and deficiency is rare in Africans (5 %) when using a cut-off value < 30 nmol/l to define deficiency.
Mithal et al. ( Reference Mithal, Wahl and Bonjour 10 ) reported that 25(OH)D concentrations below 75 nmol/l are prevalent in every region of the world, while concentrations < 25 nmol/l are most common in South Asia and the Middle East. The definition of vitamin D deficiency and insufficiency and assay methodology vary across studies, which complicates comparisons. However, our findings of increased prevalence of vitamin D insufficiency in the Asian-Indian population are in keeping with Mithal's findings.
Ethnicity has consistently been reported to be related to circulating 25(OH)D concentrations in adults( Reference Shea, Houston and Tooze 34 , Reference Forrest and Stuhldreher 35 ), and a high prevalence of hypovitaminosis D has been reported by several Indian studies( Reference Arya, Bhambri and Godbole 36 – Reference Marwaha, Tandon and Garg 38 ) as well as by studies carried out in Indian subjects in the UK( Reference Kift, Berry and Vail 39 ). The differences observed in 25(OH)D concentrations between the two ethnic groups in the present study probably reflect differences in diet, clothing and sun exposure.
Mean 25(OH)D concentrations in the African population were higher than those in the Cape Town population( Reference Charlton, Labadarios and Lombard 32 ). This may be because of differences in latitude and subject age as well as assays used to measure 25(OH)D concentrations. Concentrations were also higher than those observed in non-Hispanic Blacks and Mexican-Americans from the USA( Reference Scragg, Sowers and Bell 40 ), but much lower than those observed in the indigenous populations of East Africa( Reference Luxwolda, Kuipers and Kema 41 ). It appears that urban Africans have lower 25(OH)D concentrations than rural individuals belonging to the older age groups (>50 years)( Reference Kruger, Kruger and Wentzel-Viljoen 12 ).
In spite of the fact that Johannesburg experiences between 8 and 9 h of sunshine throughout the year, we observed a clear seasonal variation in 25(OH)D concentrations in both ethnic groups, with autumn being a very significant positive predictor of 25(OH)D concentrations in both Asian-Indians and Africans. The concentrations of 25(OH)D were lowest in subjects from whom blood samples were collected in the months of July–September (late winter and spring), indicating that sun exposure does influence 25(OH)D concentrations in both communities. Clear seasonal changes in 25(OH)D status, similar to our findings, have previously been reported in several different groups of individuals in South Africa such as the elderly with fractures( Reference Pettifor, Ross and Solomon 26 ) and HIV-positive and -negative individuals from Cape Town( Reference Martineau, Nhamoyebonde and Oni 7 ) as well as healthy African and Caucasian children living in Johannesburg( Reference Poopedi, Norris and Pettifor 11 ). In the present study, the sun exposure score was a predictor of 25(OH)D concentrations in the African population but not in the Asian-Indian population, in which females were mostly veiled. Using univariate regression analysis, weekly sun exposure score was found to be a significant predictor of 25(OH)D concentrations in the Asian-Indian population. However, after adjusting for age and sex, weekly sun exposure score was no longer a significant predictor of 25(OH)D concentrations in the Asian-Indian population. The sun exposure score comprises time spent outdoors as well as the extent of clothing coverage. It is possible that the time of day and the nature of time spent outdoors (e.g. physical activity) are also important for the determination of UV exposure, and these were not assessed in the present study. Together, these findings suggest that seasonal variation in 25(OH)D concentrations observed among the African and Asian-Indian subjects from Johannesburg is probably a result of both the time spent outdoors and the amount of clothing worn.
There was no significant association of 25(OH)D status with age in either group, while male sex was a predictor of higher 25(OH)D concentrations in the Asian-Indian population. We were unable to determine the reasons for this difference, but it is likely to be related to cultural practices such as wearing of the veil.
When total body fat and visceral and subcutaneous fat were included in the same regression model, neither total body fat nor body fat distribution was predictive of 25(OH)D concentrations in either group. We could find only one other study that has examined the relationship between body fat distribution and 25(OH)D concentrations in Asian-Indians( Reference Sulistyoningrum, Green and Lear 15 ), and our findings are in contrast to the findings reported by Sulistyoningrum et al. ( Reference Sulistyoningrum, Green and Lear 15 ). They demonstrated a negative association between visceral fat and 25(OH)D concentrations. The reasons for this difference are not known. However, there are major differences between the two studies: the Asian-Indian population in Johannesburg is quite homogenous, having migrated from India, whereas the cohort described by Sulistyoningrum et al. ( Reference Sulistyoningrum, Green and Lear 15 ) originated in India, Pakistan, Bangladesh, Sri Lanka and Nepal. VAT and serum vitamin D concentrations were each measured in the two studies by different techniques; the sample size in the present study (343 v. 192) was bigger. The mechanisms by which circulating concentrations of 25(OH)D are influenced by adipose tissue mass (or vice versa) are not fully understood. However, it is known that adipose tissue acts as a large depot for vitamin D( Reference Rosenstreich, Rich and Volwiler 42 ), which may in turn significantly affect 25(OH)D bioavailability( Reference Wortsman, Matsuoka and Chen 43 ). Furthermore, serum 25(OH)D concentrations of obese individuals exhibit a reduced response to UV-B radiation( Reference Wortsman, Matsuoka and Chen 43 ) and oral vitamin D supplementation( Reference Gallagher, Yalamanchili and Smith 44 ) when compared with those of non-obese individuals. It is interesting to note that data from the National Health and Nutrition Examination Survey III suggest that the relationship between body fat and 25(OH)D concentrations is stronger in White people than in Black people( Reference Looker 45 ).
VAT is strongly associated with cardiometabolic diseases( Reference Britton, Massaro and Murabito 46 ). In South Africa, the mortality due to CVD and the prevalence of type 2 diabetes are higher in Asian-Indians than in Africans( Reference Steinberg, Balfe and Kustner 47 ). However, in the present study, the levels of VAT were found to be similar in these two population groups. This suggests that factors other than VAT are important contributors to cardiovascular risk in the Indian population.
Ca supplementation was positively predictive of 25(OH)D concentrations in the Asian-Indian subjects, while vitamin D supplementation was not. This discrepancy may be because of the presence of vitamin D in many Ca supplements, but the amount was not specifically quantified in the present study. Significantly more Asian-Indian participants than African participants were on Ca and vitamin D supplementation. We postulate that this may be due to differences in educational and socio-economic status. Dietary vitamin D intake was low because vitamin D content in most foods is naturally low and our estimates of vitamin D intake did not include the contribution from supplements. Consequently, the lack of a relationship between dietary vitamin D intake and serum 25(OH)D concentrations is not surprising and indicates sun exposure to be the major determinant of vitamin D status in these populations. In the present study, dietary Ca intake in both ethnic groups was found to be much lower than the 1000 mg/d intake level recommended by the National Osteoporosis Foundation of South Africa. Low Ca intake could result in poor vitamin D status because of increased 25(OH)D metabolism. Despite the low dietary Ca and vitamin D intake, the plasma concentrations of 25(OH)D were much higher in the African population than in the Asian-Indian population. This highlights the important contribution of sun exposure to 25(OH)D concentrations in this part of the world.
The concentrations of PTH were not significantly different between the ethnic groups, despite a high proportion of the Asian-Indian subjects having vitamin D deficiency. Attempts have been made to define optimal vitamin D status for bone health based on the relationship between PTH and 25(OH)D concentrations( Reference Dawson-Hughes, Harris and Krall 48 ), and this relationship appears to vary by race( Reference Gutierrez, Farwell and Kermah 49 – Reference Ginde, Liu and Camargo 51 ). Thus, PTH concentrations in African-Americans are maximally suppressed at lower 25(OH)D concentrations compared with those in Whites( Reference Gutierrez, Farwell and Kermah 49 ). The present study demonstrates that the significantly lower 25(OH)D concentrations in Asian-Indians compared with those in Africans occur in the presence of similar PTH concentrations. This finding further indicates that the relationship between 25(OH)D and PTH concentrations varies by race and that in terms of bone health optimal 25(OH)D concentrations may be different in each of these two population groups. However, further studies in which bone mineral density is measured in both ethnic groups and correlated with serum PTH and 25(OH)D concentrations are required to confirm this.
Our models explained between 16 and 17 % of the variance in 25(OH)D concentrations, which is comparable to data obtained from previous studies( Reference Tran, Armstrong and McGeechan 52 – Reference Freedman, Cahoon and Rajaraman 54 ). Thus, McDonnell et al. ( Reference McDonnell, French and Heaney 53 ) analysed data on non-food factors affecting vitamin D status and demonstrated that these explained only 13 % of the variance in total 25(OH)D concentrations. Similarly, analysis of potential determinants of 25(OH)D in the US Radiologists Technologists Study has revealed that despite detailed data on UV radiation exposure being available, only 25 % of the variance in 25(OH)D concentrations is explained( Reference Freedman, Cahoon and Rajaraman 54 ). Other factors that contribute to variance include skin colour( Reference Farrar, Kift and Felton 55 ), physical activity( Reference Freedman, Cahoon and Rajaraman 54 ) and genetic factors( Reference Ahn, Yu and Stolzenberg-Solomon 56 ), none of which was assessed in the present study.
The strengths of the present study include the large sample size, the method used to measure plasma 25(OH)D concentrations, the multi-ethnic nature of the study participants, the determination of total body fat and body fat distribution, and the assessment of a large number of variables that are thought to influence vitamin D status. However, the present study has a number of limitations. First, due to the cross-sectional nature of the study, we could draw inferences as to the determinants, but could not definitively determine the causative nature of any associations. Second, we did not quantify vitamin D or Ca supplements and could therefore not accurately assess the contribution of these supplements to 25(OH)D concentrations. Furthermore, the dietary intake of Ca and vitamin D was limited to their quantification in fish, eggs and dairy products, but not in meat. We also did not assess physical activity that might influence the dermal synthesis of vitamin D and VAT and SCAT were determined by ultrasound and not by computed tomography scanning, which is the gold standard method. However, this method has been validated in this population( Reference De Lucia Rolfe, Norris and Sleigh 21 ). The questionnaire used to assess sun exposure has been used in a number of studies( Reference Hanwell, Vieth and Cole 22 , Reference Lee, Park and Kim 23 ), but has not been validated against personal UV dosimetry or observed exposure. The limit of quantification for 25(OH)D2 was 11 nmol/l, and because of this, 25(OH)D2 was not quantifiable in 9·2 % of the participants. In South African populations, 25(OH)D2 only constitutes a small proportion of total 25(OH)D and thus its lack of detectability in some subjects had little influence on the overall vitamin D status of the two ethnic groups. Finally, the number of subjects assessed in each season was not evenly distributed throughout the year.
The present study clearly demonstrates that any policy on vitamin D supplementation should be considered in a local context. Thus, season and, by implication, differences in weather conditions, dietary supplement use and ethnicity are all strong determinants of plasma 25(OH)D concentrations, and these factors, which vary widely across countries, must be considered before deciding on supplementation programmes. Adequate vitamin D concentrations in different population groups also require further study. In the present study, almost one-third of the Asian-Indians who participated were vitamin D deficient. It is possible that this group will benefit from routine supplementation. In view of the seasonal changes observed, further consideration should be given to whether year-long supplementation is required or whether supplementation can be restricted to winter and early spring. Studies are required to determine optimal dosages. The use of Ca was found to be associated with higher 25(OH)D concentrations in Asian-Indians, but the benefits and its impact on cardiovascular health have to be assessed( Reference Bostick, Kushi and Wu 27 , Reference Bolland, Barber and Doughty 57 ). In conclusion, the present study demonstrates that there are major differences in 25(OH)D concentrations in apparently healthy adults living in Johannesburg, with the highest prevalence of 25(OH)D deficiency being observed in Asian-Indians. The important positive predictors of 25(OH)D concentrations in this population are supplementation and season.
Acknowledgements
The authors thank Dr C. Padoa for reading the manuscript as well as the staff of the Birth to Twenty Study for their assistance with data collection and the staff of the National Health Laboratory Services for helping with the analysis of all the samples.
The present study was supported by a self-initiated research grant from the Medical Research Council of South Africa and a large research grant from Carnegie Foundation. Siemens diagnostics supplied PTH kits. The funders played no role in study design, data collection and analysis, decision to publish the manuscript or preparation of the manuscript.
The authors' contributions are as follows: J. A. G. planned the study, analysed and interpreted the data, and wrote the manuscript; S. A. N. assisted with planning and study design; H. E. v. D. assisted with data analysis and interpretation and write-up; J. M. P. assisted with data interpretation and proofreading; N. J. C. analysed the data and assisted with proofreading and write-up.
None of the authors has any conflicts of interest to declare.








