In late 2011 a novel insect-transmitted orthobunyavirus was discovered at the German–Dutch border and named ‘Schmallenberg virus’ after the village were the first samples were taken [Reference Hoffmann1]. Affected adult ruminants exhibit very mild, if any, clinical signs; hence, information about the initial phase of the disease in 2011 has been scarce until now. An infection of SBV-naive cows and ewes during a critical period of pregnancy can lead to severe malformed offspring [Reference Wernike, Hoffmann and Beer2, Reference Beer, Conraths and van der Poel3]. In accordance with related pathogens such as Akabane virus or Aino virus the critical phase is presumably between days 75 and 175 of gestation in cattle and from days 30 to 50 in sheep [Reference Kirkland4, Reference Parsonson5]. Considering the first reported SBV-positive malformed lambs at the beginning of December 2011 and deformed calves around the beginning of 2012 [6, 7], SBV was most likely introduced into Europe in autumn 2011. Hitherto, there is no evidence for the presence of SBV in Europe before 2011.
In the present study, a cattle farm located ∼9 km from a holding near the city of Schmallenberg [Reference Hoffmann1] was closely monitored between May 2011 and January 2012 in the context of a tick-borne fever surveillance [Reference Nieder8]. On that farm, surrounded by forest, pastures and agricultural fields (mainly corn), 58 dairy cows (Red Holstein-Friesian cattle, Simmental cattle and hybrids of both), their female offspring and two breeding bulls were kept from the beginning of the study until January 2012; six cows were slaughtered or culled and there was no introduction of animals from outside. The dairy cows are kept outdoors between May and October; roe deer are regularly seen on the pastures. The calves are kept indoors year-round. Repellents (Bayticol®, Bayer AG, Germany) were applied at 3-week intervals starting on 15 May 2011 to eight (nos. 7, 15, 16, 23, 28, 52, 53, 61) of the 19 primiparous animals until they calved (Fig. 1), and the animals were vaccinated (against bluetongue virus) in spring 2010 for the last time. A total of six animals were either slaughtered or culled up to the end of 2011. The causes of death, i.e. infertility (cattle no. 49, 11 November), udder problems (no. 56, 21 December; no. 19, 11 November), claw problems (nos. 26 and 28, both 19 September), and paresis after calving (no. 48, 11 November) were all unrelated to symptoms associated with SBV infection [Reference Wernike, Hoffmann and Beer2, Reference Beer, Conraths and van der Poel3]. The milk yield data was collected every 4–5 weeks, and fertility indicators were not calculated. The body temperature of individual animals was monitored and once fever was detected, blood samples were taken at weekly and later bi-weekly intervals.
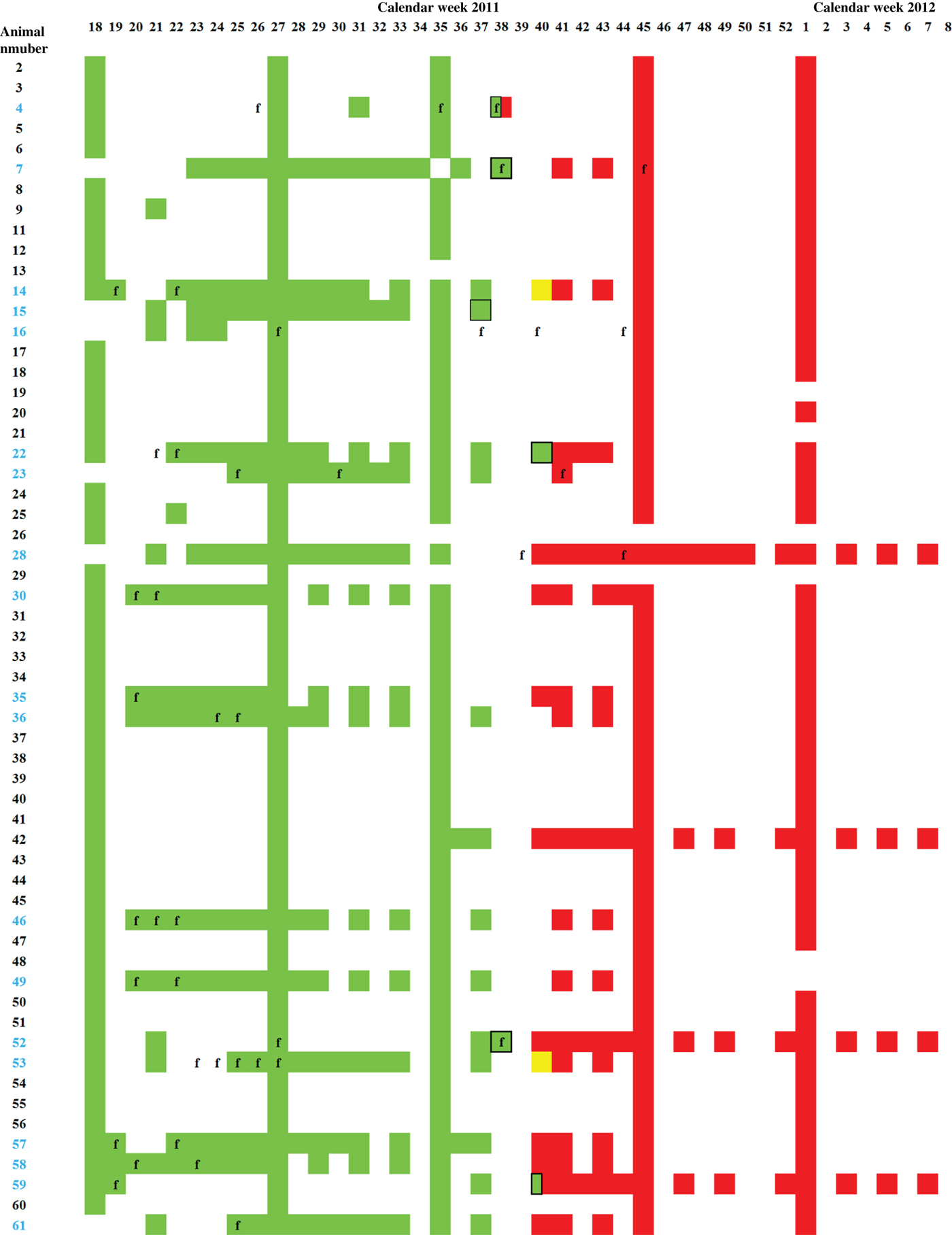
Fig. 1. Serology and real-time RT–PCR. ELISA and real-time RT–PCR results of all dairy cows kept on the monitored farm between calendar week 18 of 2011 and week 8 of 2012. Serum samples tested negative by ELISA are depicted in green, doubtful in yellow, and positive in red. PCR-positive samples are framed in black, body temperatures exceeding 39·5°C are indicated by ‘f’, and the numbers of primiparous animals are depicted in blue.
Blood samples were taken at several time points from all dairy cows (n = 58, Fig. 1). All samples were analysed by a commercially available SBV antibody ELISA (ID Screen® Schmallenberg virus Competition, IDvet, France) using the recommended cut-off of 40% relative optical density compared to the negative control. Samples taken between August and October 2011 were tested by an SBV-specific real-time reverse transcriptase–polymerase chain reaction (RT–PCR) [Reference Bilk9].
In 2011 from week 18, until calendar week 37 every tested sample was negative by SBV-specific antibody ELISA, in week 38 one out of four samples scored positive, these samples were taken from three different animals (Fig. 1). The cow sampled twice was PCR positive [quantification cycle value (Cq) 31] and negative by ELISA on 21 September and seroconverted by 25 September. Serum samples of five further animals were positive by real-time RT–PCR on 18 September (calendar week 37, Cq 31), 25 September (week 38, Cq 29 and 30, respectively) and 3 October 2011 (week 40, Cq 36 and 37, respectively) (Fig. 1). In calendar week 40, two out of 13 samples were negative by ELISA, two doubtful and nine positive, and from week 41 onwards SBV-specific antibodies were detectable in all tested serum samples (Fig. 2). During that period no abnormalities such as decreased milk yield or diarrhoea were observed in the dairy cows. Fever was frequently recognized during the 2011 season in at least 18 of the animals (see Fig. 1), and was immediately followed by blood analyses. This is shown in Figure 1 by the weekly sampling of most of these animals followed by a bi-weekly sampling and respective analyses of the blood and the serum. In three animals (nos. 4, 7, 52) the onset of fever correlated with the retrospectively diagnosed SBV infection. Until summer 2012 no fetal abnormalities were seen.

Fig. 2. Percentage of samples positive by ELISA during the course of 2011. Doubtful results were considered as seropositive.
In summer and autumn 2011, an unidentified disease in dairy cattle, now known to be SBV infection, was reported; clinical signs included a short period of fever, decreased milk yield, or diarrhoea. A robotic milking system that records daily milk yield data was not installed, but the comparison of the average milk yield between week 36 (7 September) and week 43 (24 October) did not differ, indicating no major effect of SBV infection on average milk yield in this herd. The body temperature of individual animals was monitored. The onset of fever directly coincided in three animals (animal nos. 4, 7, 52) with the period of SBV infection of the herd suggesting that SBV infection of all other animals occurred without initial fever. Interestingly, an infection with Anaplasma phagocytophilum was diagnosed in cow no. 52 in week 37, with a real-time RT–PCR-positive test for SBV the following week (week 38). Taken together the SBV infection in this herd did not cause overt clinical signs. Because the herd was subject to strict health monitoring, overlooking of mild clinical symptoms seems most unlikely, but cannot be entirely excluded. Furthermore, premature or stillbirth or the birth of malformed calves was not observed, even though at the end of September 2011, 12 of the tested cows were pregnant between days 75 and 175 of gestation, which is the presumed critical period of pregnancy [Reference Wernike, Hoffmann and Beer2].
The short viraemia of a few days observed after experimental inoculation of cattle with SBV [Reference Hoffmann1, Reference Wernike10] may be assumed after a field infection. In the present study, six cattle were PCR positive at one sampling day; in all other animals SBV genome was not detectable at any time despite continuous sampling. For example, animal no. 52 tested positive by real-time RT–PCR in calendar week 38, but negative 1 week prior to that and at 2 weeks thereafter, confirming the short period of viraemia described previously [Reference Hoffmann1, Reference Wernike10]. Considering the short viraemia and the limitation in clinical observations, serological methods seem to be most suitable for disease investigations. However, the viraemia detected in some animals of the herd may have contributed to the quick spread of the infection within the herd. First SBV-specific antibodies are detectable between 10 days and 3 weeks after experimental infection [Reference Wernike10], and antibodies to Akabane virus may be detectable 4–5 days after viraemia for the first time [Reference St George, Standfast and Cybinski11]. According to this, SBV-specific antibodies were present 1–2 weeks after positive PCR results; one animal even seroconverted within 4 days.
So far, SBV-specific antibodies have not been detected in European livestock before 2011 [Reference Conraths, Peters and Beer12], and in the present study every animal was seronegative until autumn. Therefore, the introduction of SBV before 2011 in Europe appears unlikely. Thereafter, a high seroprevalence of about 70% to nearly 100% was observed in dairy cattle and sheep in the focus of the 2011 affected area [Reference Meroc13, Reference Elbers14]. After a first exposure of cattle to Akabane virus a similarly high prevalence is observed [Reference Kirkland, Barry and Macadam15]. Accordingly, every animal tested in the present study developed SBV-specific antibodies after the introduction of the virus into the herd.
Unfortunately, previous SBV seroprevalence studies started after November 2011; consequently, the information about the course of the disease within a herd is scarce. In the present study, the first animals seroconverted at the end of September, the main vector season. Starting in mid-October every tested sample was positive by ELISA suggesting a highly effective transmission by the insect vectors involved, probably Culicoides biting midges.
In conclusion, the present study confirms previous evidence for the first entry of SBV in Europe in autumn 2011. After an exposure during the first vector season SBV spread rapidly and efficiently within a herd and the data allow further insights into SBV epidemiology.
ACKNOWLEDGEMENTS
We are grateful to Anja Landmesser for excellent technical assistance. This study was supported by the German Federal Ministry of Food, Agriculture and Consumer Protection and the European Union as outlined in Council Decision 2012/349/EU regarding a financial contribution by the Union for studies on Schmallenberg virus.
DECLARATION OF INTEREST
None.






