Helicobacter pylori infection has been classified a definite human carcinogen for almost two decades and is well accepted as the single most important risk factor for non-cardia gastric cancer(1, 2). Although the prevalence of infection has been decreasing in many of the more economically developed countries(Reference Asfeldt, Straume and Steigen3, Reference Gause-Nilsson, Gnarpe and Gnarpe4), it was estimated to be responsible for nearly one-third of the 2 million cases of cancer occurring worldwide due to infections in 2008(Reference de Martel, Ferlay and Franceschi5).
H. pylori infection is acquired mainly during childhood and adolescence(Reference Koch, Krause and Krogfelt6–Reference Oleastro, Pelerito and Nogueira8); once obtained, and in the absence of a specific treatment, it can persist for decades(Reference Sherman9). Therefore, understanding the role of modifiable exposures that may be targeted to decrease the rate of H. pylori infection during childhood is of key importance to prevent its occurrence. Factors that promote interpersonal contact or are associated with poor hygienic conditions, including being born in a setting with a high prevalence of infection(Reference Kivi, Johansson and Reilly10), having parents with a low education level(Reference Malaty, Logan and Graham11), sharing a room with other subjects(Reference McCallion, Murray and Bailie12) or attending a child-care institution(Reference Bastos, Carreira and La Vecchia13), have been consistently associated with H. pylori infection in the early years of life. Breast-feeding has long been recognized as protective against gastrointestinal and respiratory diseases(Reference Quigley, Kelly and Sacker14, Reference Wright, Bauer and Naylor15), and a role in the infection with H. pylori may be postulated.
A previous systematic review including sixteen studies suggested a protective effect of breast-feeding in middle- and low-income countries(Reference Chak, Rutherford and Steinmaus16). However, the understanding of the relationship between breast-feeding and H. pylori infection may be improved by taking into account more detailed and accurate definitions of the exposure. Furthermore, a set of additional studies were published since the previous meta-analysis, allowing an update of the existing evidence on this topic.
Therefore, we conducted a new systematic review and meta-analysis to quantify the association between breast-feeding and H. pylori infection, among children and adolescents.
Methods
Search strategy
We searched MEDLINETM and ScopusTM up to January 2013 to identify studies addressing the association between breast-feeding and H. pylori infection in childhood or adolescence. The PubMedTM and ScopusTM search expressions, and the systematic review flowchart, are presented in Fig. 1 according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statement(Reference Moher, Liberati and Tetzlaff17). The literature search was further complemented by backward citation tracking among the articles considered eligible for the systematic review.

Fig. 1 Systematic review flowchart according to the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Statement(Reference Moher, Liberati and Tetzlaff17). *Four studies(Reference Nguyen, Nguyen and Phung27, Reference Moraes and da Silva32, Reference Dore, Malaty and Graham35, Reference Daugule, Rumba and Lindkvist50) provided data to quantify the association between both having been breast-fed and having been breast-fed for 4–6 months and Helicobacter pylori infection
Selection of the studies
The studies were assessed independently by two researchers (H.C. and B.P. or H.C. and A.B.) in three consecutive steps to determine their eligibility; disagreements were discussed and resolved by consensus or involving a third researcher (N.L.).
In step 1, the studies were evaluated considering only the information presented in the title and abstract. When the abstract was not available, the study was further assessed, except when the title provided enough information to unequivocally exclude it. The full texts of the articles selected for step 2 were read to evaluate their eligibility and adequacy for data extraction; in step 3 the studies were re-evaluated to determine their eligibility for meta-analysis.
We excluded studies according to the following a priori defined criteria: (i) studies with full text not written in English, French, Italian, Polish, Portuguese or Spanish; (ii) reports not involving humans (e.g. in vitro studies); (iii) review articles, editorials, methodological studies, case reports or comments; (iv) studies in which the sample selection was dependent on the risk of H. pylori infection and therefore not expected to represent the general population (e.g. children undergoing endoscopy for diagnostic procedures); (v) studies not providing data on the association between breast-feeding and H. pylori infection; (vi) studies assessing the H. pylori infection status only in adults; and (vii) duplicate reports of the same study (data could be extracted from one or more of the multiple reports to obtain the most complete information).
Data extraction
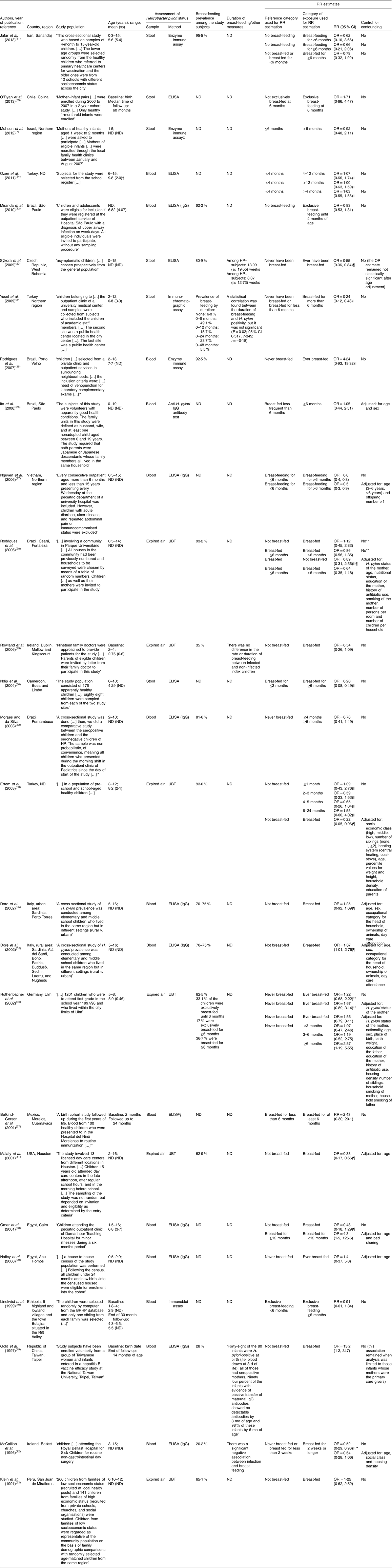
The following data were extracted from the original reports: (i) year of publication; (ii) country and region where the study was conducted; (iii) study design; (iv) sample characteristics (sample size and age distribution); (v) methods used to determine the H. pylori infection status; (vi) exposure to breast-feeding, namely regarding its duration and exclusiveness; and (vii) relative risk (RR) estimates, namely risk ratios, incidence rate ratios or odds ratios, preferably adjusted for the larger number of potential confounders, or the necessary information to compute them, along with the corresponding precision estimates. Specific estimates for exclusive and non-exclusive breast-feeding or different durations of exposure were extracted whenever available.
For the studies providing data for age groups including adults in addition to children and/or adolescents (e.g. 10–29 years), we computed the mid-point year and excluded the data when it was higher than 18 years.
The discrepancies in the data extracted independently by two reviewers (H.C. and B.P. or H.C. and A.B.) were discussed and resolved by consensus, or involving a third researcher (N.L.).
Meta-analysis
The DerSimonian and Laird method was used to compute summary estimates of the association between breast-feeding and H. pylori infection, and respective 95 % confidence intervals. Heterogeneity was quantified using the I 2 statistic(Reference Higgins and Thompson18).
Stratified analyses according to the characteristics of the populations and methodological specificities with potential impact on the internal or external validity of the results (economic development of the countries where the investigations were conducted(19), age of the participants, adjustment for the potential confounding effect of socio-economic status, method used to assess the H. pylori infection status, prevalence of H. pylori infection in the non-exposed participants, prevalence of breast-feeding) were conducted to identify factors associated with heterogeneous results.
Funnel plots and the Egger’s regression asymmetry test were used for assessment of ‘small studies effects’(Reference Sterne, Gavaghan and Egger20). The statistical analysis was performed using the STATA® statistical software package version 9·2.
No review protocol was registered.
Results
We identified thirty-eight studies eligible for the systematic review(Reference Muhsen, Jurban and Goren7, Reference Kivi, Johansson and Reilly10–Reference McCallion, Murray and Bailie12, Reference Thomas, Austin and Dale21, Reference Ndip, Malange and Akoachere30, Reference Moraes and da Silva32–Reference Vivatvakin, Theamboonlers and Semakachorn55) (Fig. 1 and Appendices 1 and 2). The studies involved samples of children and adolescents, aged 0 to 19 years, recruited in twenty-seven countries/regions, including four low-income(Reference Thomas, Austin and Dale21, Reference Lindkvist, Enquselassie and Asrat40, Reference Hestvik, Tylleskar and Kaddu-Mulindwa41, Reference Mahalanabis, Rahman and Sarker46, Reference Bhuiyan, Qadri and Saha47), thirteen middle-income(Reference Miranda, Machado and Silva22, Reference Yucel, Sayan and Yildiz24–Reference Rodrigues, Queiroz and Braga28, Reference Ndip, Malange and Akoachere30, Reference Moraes and da Silva32–Reference Ozen, Furman and Berber34, Reference Belkind-Gerson, Basurto and Newton37–Reference Naficy, Frenck and Abu-Elyazeed39, Reference Siai, Ghozzi and Ezzine42, Reference Glynn, Friedman and Gold44, Reference Gold, Khanna and Huang49–Reference O’Ryan, Rabello and Cortes53, Reference Vivatvakin, Theamboonlers and Semakachorn55) and ten high-income countries/regions(Reference Muhsen, Jurban and Goren7, Reference Kivi, Johansson and Reilly10–Reference McCallion, Murray and Bailie12, Reference Sýkora, Siala and Varvarovska23, Reference Rowland, Daly and Vaughan29, Reference Dore, Malaty and Graham35, Reference Rothenbacher, Bode and Brenner36, Reference Przybyszewska, Bielanski and Fyderek43, Reference Tindberg, Blennow and Granstrom45, Reference Okuda, Miyashiro and Koike48, Reference Gold, Khanna and Huang49, Reference Rothenbacher, Inceoglu and Bode54). Most studies had a cross-sectional design and eight were cohort studies(Reference Belkind-Gerson, Basurto and Newton37, Reference Naficy, Frenck and Abu-Elyazeed39, Reference Lindkvist, Enquselassie and Asrat40, Reference Glynn, Friedman and Gold44, Reference Tindberg, Blennow and Granstrom45, Reference Bhuiyan, Qadri and Saha47, Reference Gold, Khanna and Huang49, Reference O’Ryan, Rabello and Cortes53).
Thirteen studies did not provide information to compute an RR estimate for the association between breast-feeding and H. pylori infection(Reference Kivi, Johansson and Reilly10, Reference Thomas, Austin and Dale21, Reference Hestvik, Tylleskar and Kaddu-Mulindwa41–Reference Okuda, Miyashiro and Koike48, Reference Daugule, Rumba and Lindkvist50, Reference Rothenbacher, Inceoglu and Bode54, Reference Vivatvakin, Theamboonlers and Semakachorn55) (Appendix 1). From these, eight referred a lack of association between breast-feeding and H. pylori infection, although the point estimates were not provided(Reference Kivi, Johansson and Reilly10, Reference Hestvik, Tylleskar and Kaddu-Mulindwa41, Reference Przybyszewska, Bielanski and Fyderek43–Reference Tindberg, Blennow and Granstrom45, Reference Bhuiyan, Qadri and Saha47, Reference Daugule, Rumba and Lindkvist50, Reference Rothenbacher, Inceoglu and Bode54). One study reported that the ‘percentage of breastfeeding in the population in Korat was 61·25 %; the selected group of seropositive children had 51 % of exclusive breastfeeding for more than 6 months and 21·5 % the seropositive children had a history of breastfeeding for less than 6 months’(Reference Vivatvakin, Theamboonlers and Semakachorn55). One study involved a sample of children aged from 1 to 99 months and defined exposure as ‘presently receiving some breastmilk’(Reference Mahalanabis, Rahman and Sarker46), which is not comparable with the definitions of breast-feeding used in the remaining reports. Two studies compared the mean duration of breast-feeding among H. pylori-infected and non-infected subjects(Reference Siai, Ghozzi and Ezzine42, Reference Okuda, Miyashiro and Koike48), and therefore could not be considered for meta-analysis; only one study showed a shorter duration of breast-feeding among the participants who were infected. Thomas et al. (Reference Thomas, Austin and Dale21) compared the levels of IgA in the breast milk of the mothers and the infection with H. pylori in the respective children; the five children from the mothers who produced the lowest levels of IgA were infected.
Twenty-five studies(Reference Muhsen, Jurban and Goren7, Reference Malaty, Logan and Graham11, Reference McCallion, Murray and Bailie12, Reference Miranda, Machado and Silva22–Reference Ndip, Malange and Akoachere30, Reference Moraes and da Silva32–Reference Lindkvist, Enquselassie and Asrat40, Reference Gold, Khanna and Huang49, Reference Jafar, Jalil and Soheila51–Reference O’Ryan, Rabello and Cortes53), from high-, middle- and low-income countries, provided data to quantify the association between breast-feeding and H. pylori. Among those, fifteen compared breast-fed v. non-breast-fed subjects(Reference Malaty, Logan and Graham11, Reference McCallion, Murray and Bailie12, Reference Miranda, Machado and Silva22, Reference Sýkora, Siala and Varvarovska23, Reference Rodrigues, Corvelo and Ferrer25, Reference Rodrigues, Queiroz and Braga28, Reference Rowland, Daly and Vaughan29, Reference Ertem, Harmanci and Pehlivanoglu33, Reference Dore, Malaty and Graham35, Reference Rothenbacher, Bode and Brenner36, Reference Omar, Ibrahim and Sarkis38, Reference Naficy, Frenck and Abu-Elyazeed39, Reference Gold, Khanna and Huang49, Reference Jafar, Jalil and Soheila51, Reference Klein, Graham and Gaillour52) and twelve compared subjects breast-fed for 4–6 months v. never breast-fed or breast-fed for less than 4–6 months(Reference Muhsen, Jurban and Goren7, Reference Yucel, Sayan and Yildiz24, Reference Ito, Oba-Shinjo and Shinjo26–Reference Rodrigues, Queiroz and Braga28, Reference Ndip, Malange and Akoachere30, Reference Moraes and da Silva32–Reference Ozen, Furman and Berber34, Reference Rothenbacher, Bode and Brenner36, Reference Belkind-Gerson, Basurto and Newton37, Reference Jafar, Jalil and Soheila51). Two studies specifically addressed the exclusive breast-feeding until the age of 6 months(Reference Lindkvist, Enquselassie and Asrat40, Reference O’Ryan, Rabello and Cortes53) (Appendix 2).
H. pylori infection according to history of breast-feeding (ever v . never)
Having been breast-fed was not significantly associated with H. pylori infection in either high-income (summary RR=0·85; 95 % CI 0·54, 1·34; I 2=79·1 %) or middle-income countries (summary RR=0·87; 95 % CI 0·57, 1·32; I 2=34·4 %). The results were heterogeneous, possibly reflecting a large inter-study variation in the duration of breast-feeding, since the prevalence of breast-feeding and the age range of the participants varied widely across studies (Fig. 2).

Fig. 2 Meta-analyses of studies evaluating the association between ever being breast-fed and Helicobacter pylori infection. Relative risk (RR) estimates and 95 % confidence intervals of H. pylori infection according to economic development of the countries where the investigations were conducted(19). For each study, the black diamond indicates the best estimate, the size of the grey square indicates the study’s weight in the analysis (weights are from random-effects analysis) and the horizontal line represents the 95 % CI. The centre of the open diamond indicates the summary estimate of the RR and its width represents the 95 % CI of the summary RR estimate. General abbreviations: SES, socio-economic status; Prev., prevalence (%); HP+, H. pylori-infected; BF, breast-feeding; NA, not available. Abbreviations for countries: BRA, Brazil; TWN, Taiwan, Republic of China; CZE, Czech Republic; DEU, Germany; EGY, Egypt; GBR, United Kingdom; IRL, Ireland; IRN, Islamic Republic of Iran; ITA, Italy; PER, Peru; TUR, Turkey. Abbreviations for tests: SA, test based on the detection of stool antigens; SI, test based on serum immunology; UBT, urea breath test. *In the World Bank statistics, Taiwan, Republic of China, is not listed as a separate country. However, for most indicators, Taiwan’s data are not added to the data for China, but it is added to the world aggregate and the high-income countries aggregate. Therefore, Taiwan was included along with other high-income settings
The visual inspection of the funnel plot did not suggest the occurrence of publication bias (Fig. 3). This is corroborated by the Egger’s asymmetry test (P=0·84).

Fig. 3 Funnel plot of studies evaluating the association between breast-feeding and Helicobacter pylori infection: (a) ever breast-feeding v. never; (b) breast-feeding for 4–6 months v. less than that. Studies were plotted with their relative risk (RR) estimate on the x-axis (log scale) and the corresponding standard error of the RR along the y-axis; pseudo 95 % confidence limits are represented by dashed lines
H. pylori infection according to duration of breast-feeding
Only two studies provided data to evaluate the association between being breast-fed for 4 months or more v. never breast-fed or breast-fed for less than 4–6 months in high-income settings(Reference Muhsen, Jurban and Goren7, Reference Rothenbacher, Bode and Brenner36). The overall RR estimate was 1·56 (95 % CI 0·57, 4·26; I 2=68·3 %; Fig. 4). The single study that provided an RR estimate adjusted for confounders yielded an OR of 2·57 (95 % CI 1·19, 5·55).

Fig. 4 Meta-analyses of studies evaluating the association between the duration of breast-feeding (<4–6 months v. >4–6 months) and Helicobacter pylori infection. Relative risk (RR) estimates and 95 % confidence intervals of H. pylori infection according to economic development of the countries where the investigations were conducted(19). For each study, the black diamond indicates the best estimate, the size of the grey square indicates the study’s weight in the analysis (weights are from random-effects analysis) and the horizontal line represents the 95 % CI. The centre of the open diamond indicates the summary estimate of the RR and its width represents the 95 % CI of the summary RR estimate. General abbreviations: SES, socio-economic status; Prev., prevalence (%); HP+, H. pylori-infected; BF, breast-feeding; NA, not available. Abbreviations for countries: BRA, Brazil; CMR, Cameroon; DEU, Germany; IRN, Islamic Republic of Iran; IRS, Israel; MEX, Mexico; TUR, Turkey; VNM, Vietnam. Abbreviations for tests: SA, test based on the detection of stool antigens; SI, test based on serum immunology; UBT, urea breath test. *Study comparing those who were breast-fed for more than 6 months with those breast-fed for less than 2 months. A sensitivity analysis excluding this study yielded an overall RR estimate of 0·80 (95 % CI 0·54, 1·19; I 2=65·8 %)
The combined results of the ten studies conducted in middle-income settings showed a summary RR of 0·66 (95 % CI 0·44, 0·98; I 2=65·7 %). The summary RR was non-significant when considering the adjustment for potential confounding effect of socio-economic factors (adjusted: summary RR=0·77; 95 % CI 0·48, 1·20; I 2=40·5 %; unadjusted: summary RR=0·58; 95 % CI 0·30, 1·10; I 2=76·3 %), or the prevalence of H. pylori infection among the non-exposed subjects (using the median as cut-off; ≤43 %: summary RR=0·67; 95 % CI 0·35, 1·27; I 2=75·0 %; >43 %: summary RR=0·66; 95 % CI 0·38, 1·15; I 2=60·7 %). The three studies that used diagnostic tests based on the detection of stool antigens yielded lower RR estimates (summary RR=0·33; 95 % CI 0·15, 0·73; I 2=64·3 %), as did the seven studies with younger subjects (using the median as cut-off; ≤7 years: summary RR=0·50; 95 % CI 0·32, 0·78; I 2=56·7 %; >7 years: summary RR=1·09; 95 % CI 0·77, 1·55; I 2=0·0 %). The only cohort analysis showed a non-significant positive association between breast-feeding and H. pylori infection (RR=2·54; 95 % CI 0·29, 22·40), although only six out of 110 children seroconverted during the 2-year follow-up period since birth(Reference Belkind-Gerson, Basurto and Newton37).
The visual inspection of the funnel plot and the results of the Egger’s asymmetry test (P=0·82) did not suggest publication bias (Fig. 3).
H. pylori infection according to history of exclusive breast-feeding
Two studies(Reference Lindkvist, Enquselassie and Asrat40, Reference O’Ryan, Rabello and Cortes53), conducted in Ethiopia (low-income country) and in Chile (middle-income country), assessed the effect of exclusive breast-feeding for more than 6 months; the RR was 0·91 (95 % CI 0·61, 1·34) and 1·71 (95 % CI 0·66, 4·47) in the low- and middle-income setting, respectively.
Discussion
The available evidence on the relationship between breast-feeding and H. pylori infection is compatible with a protective effect in the less economically developed settings. However, only a few studies accounted for the potential confounding by socio-economic factors or assessed the effects of breast-feeding duration or exclusivity, precluding definite conclusions on this topic.
The present study updated a previous systematic review and meta-analysis conducted by Chak et al.(Reference Chak, Rutherford and Steinmaus16) and the interpretation of our findings needs to take into account the evidence that was published since then, as well as the differences in the completeness of the search strategy and options for data synthesis. The present systematic review included eighteen studies(Reference Muhsen, Jurban and Goren7, Reference Miranda, Machado and Silva22–Reference Ito, Oba-Shinjo and Shinjo26, Reference Rowland, Daly and Vaughan29, Reference Ndip, Malange and Akoachere30, Reference Moraes and da Silva32, Reference Ozen, Furman and Berber34, Reference Belkind-Gerson, Basurto and Newton37, Reference Omar, Ibrahim and Sarkis38, Reference Daugule, Rumba and Lindkvist50–Reference Vivatvakin, Theamboonlers and Semakachorn55) that were not considered in the paper published by Chak et al.(Reference Chak, Rutherford and Steinmaus16); most of the studies were published since then and two(Reference Rodrigues, Corvelo and Ferrer25, Reference Belkind-Gerson, Basurto and Newton37) were written in languages probably not considered in the previous review. However, due to our methodological options, six studies(Reference Ueda, Kikuchi and Kasugai31, Reference Mahalanabis, Rahman and Sarker46, Reference Pearce, Thomas and Campbell56–Reference Braga, Fialho and Rodrigues59) included in the previous meta-analysis were not included in our meta-analyses. The study by Suoglu et al. (Reference Suoglu, Gokce and Saglam58) was not eligible because the sample selection was not independent of the H. pylori status. The study by Braga et al. (Reference Braga, Fialho and Rodrigues59) included a sample that partially overlapped with the sample of the study by Rodrigues et al.(Reference Rodrigues, Queiroz and Braga28) and only the latter was considered in our review. The study by Mahalanabis et al. (Reference Mahalanabis, Rahman and Sarker46) defined the exposure as ‘presently receiving some breast milk’ although it also included children old enough for not being breast-fed for a long time and was therefore excluded from our analyses. We also opted for not including the studies evaluating the H. pylori infection status in adulthood(Reference Ueda, Kikuchi and Kasugai31, Reference Pearce, Thomas and Campbell56, Reference Fall, Goggin and Hawtin57), as the larger the lag between the exposure to breast-feeding and the assessment of infection status, the more likely it is that the RR estimates reflect the effect of other factors in addition to breast-feeding, namely taking into account that the incidence rates may remain high throughout adolescence(Reference Bastos, Peleteiro and Pinto60).
The inclusion of a larger number of studies allowed a finer assessment of the exposure of breast-feeding. Chak et al. (Reference Chak, Rutherford and Steinmaus16) provided a summary OR estimate combining the results of all eligible studies, regardless of the breast-feeding definition, and conducted stratified analysis according to the duration of breast-feeding (≥4 months v. <4 months or not specified). We opted for conducting two sets of analyses: (i) according to the breast-feeding status (ever breast-fed v. never breast-fed); and (ii) according to the duration of breast-feeding (≥4–6 months v. <4–6 months). Despite our efforts to combine the results from more homogeneous groups of studies, the inter-study variability in the estimates remained high. Among the studies that assessed the H. pylori status among those ever breast-fed and those who were never, the heterogeneity of the results is likely to be explained primarily by the differences implicit in the definition of ever having been breast-fed, which may include children breast-fed for one week or one year; however, the original reports did not provide information to account for these methodological aspects in our analyses. This depicts the need for standardized breast-feeding definitions to be used for the collection and description of data on this topic(Reference Labbok and Starling61). In 1988, the Interagency Group for Action on Breastfeeding(Reference Labbok and Krasovec62) recognized that the term ‘breast-feeding’ is not enough to accurately describe its numerous variations. Specifically, it is required to distinguish between full and partial breast-feeding, and between the different levels of partial breast-feeding(Reference Labbok and Krasovec62).
Our results suggest that having been breast-fed for 4–6 months is associated with a lower risk of H. pylori infection only in middle-income countries. We may hypothesize that in the latter settings children who are being breast-fed may present a substantially better nutritional status and therefore present more resistance to infections. Also, children whose mothers had breast milk with higher levels of anti-H. pylori IgA had a lower risk of H. pylori infection, compared with those whose mothers had lower levels(Reference Thomas, Austin and Dale21). Furthermore, breast-feeding may protect against the acquisition of the infection by acting as a natural antibiotic, as bovine lactoferrin was shown to inhibit the growth of H. pylori (Reference Dial, Hall and Serna63–Reference Dial and Lichtenberger65); lactoferrin is much more abundant in breast milk than it is in cow’s milk. Another component of breast milk, κ-casein, was shown to play a role in the inhibition of H. pylori adhesion to gastric mucosa(Reference Hamosh66).
Although similar results were obtained when considering crude RR estimates with those adjusted for the potential confounding by socio-economic factors, this is a methodological aspect of major importance and a sound assessment of this relationship requires the control of these confounders. The relationship between low socio-economic status and H. pylori infection is well known(Reference Ford and Axon67, Reference Graham, Malaty and Evans68). Breast-feeding is also influenced by these factors, although the relationship may vary with time and across settings with different economic and cultural background(Reference Imdad, Yakoob and Bhutta69–Reference Lunet and Barros71).
There was no association between breast-feeding and H. pylori infection when only the studies including older children were considered for analysis, which may reflect a lack of longer-term effects of breast-feeding, or that more important risk factors exert their effects after the cessation of breast-feeding.
Conclusion
In conclusion, our results suggest a protective effect of breast-feeding in economically less developed settings. However, further research is needed, with a finer assessment of the exposure to breast-feeding and infection status, as well as a careful control of confounding, before definite conclusions can be reached.
Acknowledgements
Financial support: This study was funded by a grant of the Fundação para a Ciência e a Tecnologia (grant number PTDC/SAU-EPI/122460/2010). The Fundação para a Ciência e a Tecnologia had no role in the design, analysis or writing of the article. Conflict of interest: None. Authorship: H.C. conceived the study and performed the collection and analyses of data under the supervision of N.L. at all stages of its implementation. A.B. and B.P. collaborated in data collection. All authors contributed to the interpretation of results and review of drafts of the manuscript, and read and approved the final submission of the manuscript.
Appendix 1 Main characteristics and results of the studies included in the systematic review but excluded from the meta-analysis

Appendix 2 Main characteristics and results of the studies included in the meta-analysis