Introduction
With the intensification of global food production, agricultural strategies have increasingly emphasized maximizing crop yields, often at the expense of other ecosystem services (ES)Reference Foley, DeFries, Asner, Barford, Bonan, Carpenter, Chapin, Coe, Daily, Gibbs, Helkowski, Holloway, Howard, Kucharik, Monfreda, Patz, Prentice, Ramankutty and Snyder1. This has been accomplished, in part, by replacing the original native vegetation with simplified systems dominated by a single or a few crop species, and by favoring annual over perennial species. Although this trend has led to the more than doubling of global food production since the 1950s2, maintaining these productivity levels over time demands large amounts of external inputs such as fertilizers, pesticides, irrigation and fossil fuelsReference Tilman, Cassman, Matson, Naylor and Polasky3. Concomitantly, many of the ES provided by diverse, native plant communities—including regulating (e.g., water supply and quality, climate and pest control), supporting (nutrient cycling, soil formation and pollination), and cultural (spiritual, recreation, education, medicine, etc.) services—are being lost or severely diminishedReference Power4. As the global society increasingly confronts the consequences of these losses—acutely evidenced by expanding hypoxic zones, declining water quality, increased incidence of severe flooding and drought, and impoverished biodiversity—there is a growing urgency for developing multifunctional agricultural landscapes that provide diverse ES in addition to provisioning services such as food, fiber, fuel and fodder productionReference Boody, Vondracek, Andow, Kinke, Westra, Zimmerman and Welle5–Reference Swinton, Lupi, Robertson and Hamilton7.
One promising approach to expanding ES provided by agricultural landscapes is through ‘perennialization’, defined here as the strategic incorporation of diverse perennial plants as integral and purposeful components of agroecosystems to enhance ES benefits. This approach is based on the premise that the positive impacts of perennial plants can be magnified when perennials are targeted to landscape positions that yield disproportionately high ES benefits relative to the land area they occupy. Diverse perennial plant communities have been shown to enhance hydrologic regulationReference Gerla8, water qualityReference Duchemin and Hogue9, carbon sequestration and storageReference Zan, Fyles, Girouard and Samson10, beneficial organisms for pest control and pollinationReference Fiedler and Landis11, soil qualityReference Moonen and Barberi12 and biological functioningReference Fonte and Six13 relative to simplified cropping systems. Perennial plants can also provide a range of provisioning services to society, including food, fiber, fuel and feed, which can contribute to diversifying production and reducing risk. The societal benefits of perennialization also include cultural and social amenities, as more diverse landscapes can positively impact aesthetic, recreational, tourism and health values within local communitiesReference Milestad, Ahnstrom and Bjorklund14.
Perennialization can also provide a tool for both mitigating and adapting to climate change. Climate change poses a major threat not only to agricultural productivity but also to the capacity of agricultural systems to provide diverse ESReference Deryng, Sacks, Barford and Ramankutty15, Reference Schlenker and Roberts16. In recent decades, an emphasis of agronomic research has been the development of technologies for maintaining crop yields under future climates, for example, by increasing external inputs, crop breeding or genetic engineeringReference Challinor, Wheeler, Craufurd, Ferro and Stephenson17–Reference Motha and Baier19. However, these approaches often do not consider the importance of concomitantly sustaining the diverse ES of agricultural landscapesReference Smith and Olesen20. Increasing the amount of perennial cover in agricultural landscapes generally augments biodiversity, and more diverse ecological communities tend to have greater resilience (the ability to recover rapidly from stress), and stability (the ability to resist change or withstand stress without loss of function), relative to simplified plant communitiesReference Tilman, Knops, Wedin, Reich, Ritchie and Siemann21. Perennialization, by increasing biodiversity of both plants and biological organisms, provides one mechanism for enhancing agroecosystem resilience and stability of cropping systems and their associated ES under climate changeReference Lin22–Reference Tilman, Hill and Lehman24. With the frequency of extreme events such as drought, flooding, pest infestation and disease predicted to increase under future climatesReference Solomon, Qin, Manning, Chen, Marquis, Averyt, Tignor and Miller25, enhanced resilience and stability will be critical for maintaining the long-term sustainability of food production systemsReference Lin22, Reference DiFalco and Chavas26, Reference Lin, Perfecto and Vandermeer27.
Although multifunctionality of agricultural landscapes is not a new conceptReference Boody, Vondracek, Andow, Kinke, Westra, Zimmerman and Welle5–Reference Swinton, Lupi, Robertson and Hamilton7, studies documenting ES consequences when some portions of agricultural landscapes are not utilized to produce food, fiber or fuel, but instead are maintained under perennial vegetation to produce other, less quantifiable or marketable products, are only beginning to emergeReference Kremen, Williams, Bugg, Fay and Thorp28, Reference Ricketts, Regetz, Steffan-Dewenter, Cunningham, Kremen, Bogdanski, Gemmill-Herren, Greenleaf, Klein, Mayfield, Morandin, Ochieng and Viana29. In particular, more information is needed about how different types, amounts and locations of perennial cover influence the cost–benefit tradeoffs of different ESReference Brussaard, Caron, Campbell, Lipper, Mainka, Rabbinge, Babin and Pulleman30–Reference Robertson and Swinton33, as well as the policy structures required to facilitate changes in land management practices and market mechanismsReference Dale and Polasky31, Reference Chan, Shaw, Cameron, Underwood and Daily34. Moreover, because areas with perennial vegetation tend to support plant communities having greater species diversity relative to areas managed purely for agricultural crops, teasing apart the relative contributions of perenniality versus biodiversity to the provisioning of different ecosystems services is another challenge in perennialization research requiring greater attention.
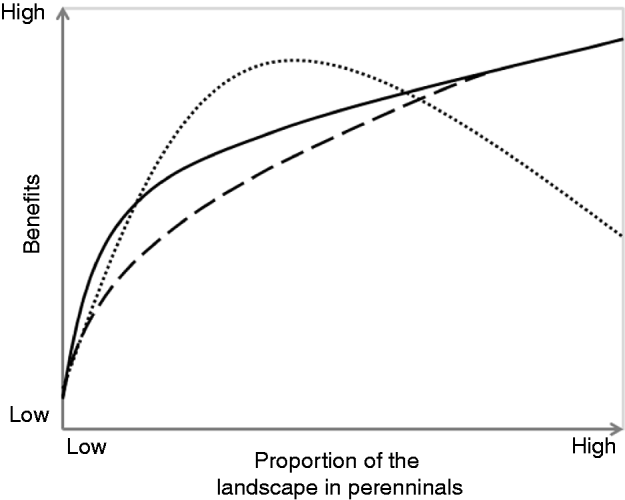
In this paper, we argue that redesigning agroecosystems based on perennialization principles can dramatically improve the capacity of agricultural landscapes to provide valuable ES to society, while simultaneously producing sufficient amounts of food, fiber, fuel and bioenergy (Fig. 1). In this paper, we discuss how targeted integration of appropriate mixtures of perennial plants into agricultural landscapes offers a common unifying strategy for augmenting diverse ES, balancing food production with conservation, and enhancing agroecosystem resilience and stability. We focus our discussion on the humid temperate Corn Belt of the Midwestern US, a major global food-producing region accounting for approximately 40% of the world's total production of corn (Zea mays)35. The Corn Belt provides an excellent model system for our analysis because it has experienced one of the most pronounced landscape scale conversions worldwide, with ∼70% of the pre-European native grassland vegetation replaced by monocultural annual crops of corn and soybean (Glycine max)36. Extensive drainage of natural wetlands together with intensification and mechanization of agricultural practices have accompanied the conversion processReference Dahl and Allord37. These land-use changes have severely compromised the delivery of fundamental ES to society, such as clean waterReference Goolsby and Battaglin38, Reference Jaynes, Hatfield and Meek39, odor- and toxic-free airReference Aneja, Blunden, Roelle, Schlesinger, Knighton, Niyogi, Gilliam, Jennings and Duke40, flood controlReference Knox41 and wildlife habitatReference Farrar42. Despite growing interest over the past several decades in perennial-based conservation practices in the regionReference Schulte, Liebman, Asbjornsen and Crow6, a broader valuation of the multiple ES achievable through strategic targeting of perennial vegetation is needed (Fig. 2). We complement our analysis with examples from other regions (e.g., China, Africa and Central America) that have experienced similar trends of agricultural intensification and simplification, and where significant opportunities also exist for better balancing food production and ES through perennialization. Although a detailed global comparative analysis is beyond the scope of this paper, by highlighting documented examples where perennialization has contributed on enhancing ES provisioning around the world, we aim to show the broad application of perennialization principles, stimulate cross-country exchange of experiences, and advance new ideas and thinking about future directions of perennialization research.

Figure 1. Disproportionate benefits hypothesis: perennial vegetation is expected to produce benefits disproportional to its extent within landscapes; ecosystem benefits of non targeted perennial cover (dashed line), ecosystem benefits of targeted perennial cover (solid line), and socio-economic benefits (dotted line). Examples of ecological benefits include clean water, flood control, pollination, pest suppression and outdoor recreational opportunities. Examples of social benefits include inspiration, connectedness and civic engagement.

Figure 2. A virtual agricultural landscape with perennial vegetation that is strategically integrated in varying proportions (left) to enhance the delivery of ES, as illustrated in the ‘flower’ diagrams (right); the proportion of perennial vegetation in each landscape is as follows: (a) 4%, (b) 16% and (c) 64%.
Given the wide range of potential ES provided by perennialization of agricultural landscapes, we focus our discussion on five ES considered particularly important for sustaining biophysical and human systems: hydrologic regulation and water purification, climate regulation and climate change mitigation, biotic regulation, soil quality and nutrient cycling, and provisioning services. For each ES, we synthesize the current state of knowledge, identify important gaps and research priorities, and consider how mixed perennial–annual agricultural systems in the Midwestern USA compare with other regions in the world.
Hydrologic Regulation and Water Purification
Declining hydrologic services related to water supply and quality represent a major threat to the well-being of human populations in many regions of the worldReference Scanlon, Jolly, Sophocleous and Zhang43, and have been directly linked to the expansion of intensively managed annual crops at the expense of diverse perennial vegetationReference Tilman, Fargione, Wolff, D'Antonio, Dobson, Howarth, Schindler, Schlesinger, Simberloff and Swackhamer44. In the Midwestern US, landscape-scale conversion from perennial to annual crops has resulted in significantly increased streamflow and base flowReference Schilling and Libra45, increased sedimentReference Knox46 and nutrientReference David, McLsaac, Darmody and Omonode47 delivery to streams, and the formation of hypoxic zones in the Gulf of MexicoReference Rabalais, Diaz, Levin, Turner, Gilbert and Zhang48.
Perennial plants can restore water purification and flow regulation services to these agriculturally dominated landscapes through several mechanisms. In humid temperate climates such as the Midwestern US, where excess water is often a problem, especially in the early growing season before annual crops have become established, the deeper roots and longer growing season typical of perennial vegetation results in greater total water uptake and evapotranspiration compared with annual cropsReference Anderson, Udawatta, Seobi and Garrett49, Reference Brye, Norman, Bundy and Gower50. Additionally, perennial vegetation can improve soil structure and hydraulic properties by increasing the number and size of macroporesReference Seobi, Anderson, Udawatta and Gantzer51–Reference Liebig, Johnson, Hanson and Frank53, building organic matterReference Liebig, Johnson, Hanson and Frank53, Reference Tufekcioglu, Raich, Isenhart and Schultz54 and improving soil porosityReference Hernandez-Santana, Zhou, Helmers, Asbjornsen, Kolka and Tomer55, Reference Wang56. Combined, these changes in plant water fluxes and soil properties contribute to greater soil water storage capacityReference Schilling, Jha, Zhang, Gassman and Wolter57, higher infiltration ratesReference Bharati, Lee, Isenhart and Schultz58 and reduced runoffReference Steward, Yang, Lauwo, Staggenborg, Macpherson and Welch59. Perennial grassesReference Blanco-Canqui, Gantzer, Anderson and Alberts60, Reference Helmers, Zhou, Asbjornsen, Kolka, Tomer and Cruse61, treesReference Leguedois, Ellis, Hairsine and Tongway62 and tree–grass mixturesReference Lee, Isenhart and Schultz63 have also been shown to more effectively trap and retain sediment compared with annual crops.
Critical to developing effective perennialization strategies is a sound understanding of how much of the landscape needs to be converted to perennial cover, where such conversions are best located and which species mixtures are most effective. Studies based on both modelingReference Dosskey, Helmers, Eisenhauer, Franti and Hoagland64, Reference Sahu and Gu65 and empirical measurementsReference Liu, Mang and Zhang66, Reference Zhang, Liu, Zhang, Dahlgren and Eitzel67 suggest a nonlinear relationship between the amount of perennial cover and different hydrologic services, resulting in diminishing returns beyond a certain amount. Such nonlinear relationships have been demonstrated for sediment trapping efficiencyReference Helmers, Zhou, Asbjornsen, Kolka, Tomer and Cruse61, Reference Lee, Isenhart and Schultz63, Reference Schmitt, Dosskey and Hoagland68, Reference Abu-Zreig, Rudra, Lalonde, Whiteley and Kaushik69, retention of nitrogen (N), phosphorus (P)Reference Schmitt, Dosskey and Hoagland68, Reference Mayer, Reynolds, McCutchen and Canfield70, Reference Zhou, Helmers, Asbjornsen, Kolka and Tomer71 and pesticidesReference Patty, Real and Gril72, and runoff reductionReference Hernandez-Santana, Zhou, Helmers, Asbjornsen, Kolka and Tomer55, Reference Wang56. Combined, these findings underscore the notion that relatively small amounts of perennial cover may produce significant ES benefits, while minimizing negative impacts on crop production (Fig. 1).
The location of perennial vegetation in the landscape is critical in determining the lateral and vertical movement of water through watersheds. For example, Sahu and GuReference Sahu and Gu65 used a modeling approach to show that strips occupying 10–20% of the catchment area were more efficient in reducing NO3–N when placed along the contour than along the riparian zone. Jiang et al.Reference Jiang, Anderson, Kitchen, Sadler and Sudduth73 found that perennial plants located on the backslope position showed greater improvement in hydraulic properties compared with summit and footslope positions, suggesting that targeting perennial plantings to slope positions that are most vulnerable to degradation may provide the greatest benefit–cost returns. Another important consideration is that hydrologic flows generally are not distributed uniformly along buffer strip edgesReference Dosskey, Helmers, Eisenhauer, Franti and Hoagland64, Reference Tomer, James and Isenhart74, and soil water content may also vary with topographic positionReference McGee, Peterson and Westfall75. Thus, strategically locating perennial vegetation to maximize flow interception can optimize hydrologic regulation and water purification benefitsReference Tomer, Dosskey, Burkart, James, Helmers and Eisenhauer76. Nonetheless, placement of buffer strips on the landscape under current practices is often dictated by economic factorsReference Yang and Weersink77, and more work is needed to incorporate approaches for identifying landscape positions for maximizing ES gains into policy and management decisions.
Few studies have assessed the interactive effects of both amount and location of perennial cover on the hydrologic functioning at the watershed scale. One study in central Iowa assessed the effects of varying amounts (10% or 20% of total watershed area) and locations (20% all at the bottom or 20% distributed in contour strips) of native perennial prairie vegetation on sediment and nutrient loss from experimental watershedsReference Geza, Barfield, Huhnke, Stoecker, Storm and Stevens78. During an extreme rainfall year (2010), watersheds having 100% annual crops had up to 35-fold greater sediment loss (6412 kg ha−1) compared with watersheds having perennial strips (180–314 kg ha−1)Reference Helmers, Zhou, Asbjornsen, Kolka, Tomer and Cruse61. In a relatively dry year (2009), the differences among treatments were also substantial: 4114 kg ha−1 sediment lost from watersheds having 100% crops compared with 129–173 kg ha−1 from watersheds with perennial strips. Similar results were observed for N: during a wet year (2008) 100% cropped watersheds had 8-fold greater N export (83 kg ha−1) relative to watersheds with perennial strips (8.1–12.3 kg ha−1); the difference was 6-fold during a dry year (2009). Patterns observed for P losses were even more pronounced, with 100% cropped watersheds having 11-fold and 16-fold greater P export in 2008 and 2009, respectively, compared with watersheds containing perennial strips. Annual total runoff was 1.6 times greater for watersheds with 100% crops compared with watersheds with the perennial stripsReference Hernandez-Santana, Zhou, Helmers, Asbjornsen, Kolka and Tomer55, Reference Wang56. Across all measured variables and years, watersheds with 10% perennial cover at the bottom unexpectedly performed slightly better than watersheds with 20% perennial cover distributed as contour strips, and the reason for this is believed to be due to a larger area in perennial cover at the footslope position for the watersheds with 10% perennial cover at the bottom. The 10% perennial cover at the bottom treatment had the greatest perennial cover at the footslope position rather than intermixed within the watershed so likely provided the greatest protection in reducing sediment and nutrient loss. However, over time the contour strips distributed within the watershed may provide a naturally terracing effect, which could improve the performance of this treatment. Overall, the findings from this study highlight the potential short-term (2–4 yr) benefits provided by strategically integrating small amounts (as little as 10% of the entire watershed) of perennial strips within annual cropping systems. More long-term monitoring is needed to understand the long-term functioning of these strips once they become well established.
Although we are unaware of other watershed scale field studies addressing interactions between both amount and location of perennial cover, several other approaches have been used to identify landscape locations for targeting perennial cover to maximize benefits. Geza et al.Reference Geza, Barfield, Huhnke, Stoecker, Storm and Stevens78 used the Soil and Water Assessment Tool (SWAT) to evaluate environmental and economic trade-offs between placing grass filter strips along field drains or targeting their placement to environmentally sensitive areas. While the authors found that placement of the filter strips along field drains was more cost effective, they concluded that the targeted approach would be more environmentally beneficial due to the greater potential for enhancing soil conservation. Terrain analysis has been used to identify sites with large wetness indicesReference Tomer, James and Isenhart74, and soil survey and topographic and streamflow data have been combined to develop a system for prioritizing locations for maximizing sediment trapping and surface runoff and/or shallow groundwater flow interceptionReference Tomer, Dosskey, Burkart, James, Helmers and Eisenhauer76. Geographic information systems and multiple criteria ranking procedures have been used to identify the intersection of hydrologically sensitive and pollutant source areas to develop decision-making processes for effective buffer placementReference Qiu79. While these studies have generated similar conclusions—that targeting perennial cover to key areas in agricultural landscapes maximizes environmental benefits—supporting empirical data are lacking for a range of climatic and edaphic conditions.
The selection of particular species or functional groups (e.g., trees versus grasses) can also affect the hydrologic functioning of perennial vegetation, but information is relatively scarce. Studies comparing the hydrologic functions of different species of herbaceous perennials suggest that species may vary substantially in their ability to reduce overland flowReference Wu, Huang, Teng and Sardo80 and herbicide leachingReference Belden and Coats81, Reference Smith, Putnam, Phaneuf, Lanza, Dhankher and Clark82, and to increase sediment trapping efficiencyReference Blanco-Canqui, Gantzer, Anderson and Alberts60, Reference Meyer, Dabney and Harmon83, Reference Wilson, Cruse and Burras84 and water uptakeReference Brye, Norman, Bundy and Gower85. The effectiveness of grass buffers versus mixed grass–tree buffers appears to vary, with some studies suggesting a similar capacity to remove N and PReference Duchemin and Hogue9, Reference Daniels and Gilliam86, while others report greater N and P removalReference Seobi, Anderson, Udawatta and Gantzer51, Reference Lee, Isenhart and Schultz63 and greater infiltration ratesReference Schilling, Jha, Zhang, Gassman and Wolter57, Reference Udawatta, Krstansky, Henerson and Garrett87 under mixed tree–grass riparian buffers compared with grass-only buffers. A paired watershed study found that after 9 years, grass buffer strips significantly decreased runoff by 8.4%, yet there was no significant effect of agroforestry buffer strips, relative to the controlReference Veum, Goyne, Motavalli and Udawatta88. One reason for these contrasting results may be due to the different ages of the tree-based buffers as trees require more time to fully establish before they exhibit positive effects on hydrologic functionsReference Gajic, Dugalic, Sredojevic and Tomic89. Further, research is lacking on the potential competitive interactions between trees and adjacent crops in temperate agroforestry systems, as water, nutrient and light resources available for crop growth may become more limitingReference Jose, Gillespie and Pallardy90. Overall, improved understanding is needed of how differences in functional type and species composition of perennial vegetation affect hydrologic performance to better select the most suitable mixtures for achieving specific perennialization objectives.
In other climatic regions worldwide, the presence of perennial vegetation in agricultural landscapes can enhance hydrologic services, but impacts vary widely depending on site-specific agroecosystem–climate interactions. For example, in the humid tropics, many traditional agroecosystems comprise combinations of different perennial and annual plants with high structural and compositional complexity, and are often designed to both meet local subsistence needs while simultaneously producing a few specialty high-value market cropsReference Kumar and Nair91. Two classical market-oriented production systems, shade coffee and cacao plantations, which target the production of perennial crops while maintaining relatively high native species diversity, have been shown to maintain similar hydrologic services compared with the native forestsReference Godsey and Elsenbeer92–Reference Zimmermann, Elsenbeer and De Moraes94. Hanson et al.Reference Hanson, Steenhuis, Walter and Boll93 showed that following conversion of primary forests to grassland, soils had lower saturated hydraulic conductivity and more frequent overland flow compared with soils under traditional shade-grown coffee plantations, while the latter maintained high infiltration capacities and readily conducted water vertically, similar to conditions under primary forest. However, as a result of increasing market pressures and land-use intensification, many shade-grown agroforestry systems are being converted to less diverse and more structurally homogeneous sun-grown plantationsReference Muschler and Bonnemann95, Reference Perfecto, Rice, Greenberg and Van Der Voort96 or, in some cases, to annual or short duration crops such as sugarcaneReference Lindsay and Rocha97, Reference Thomlinson, Serrano, Lopez, Aide and Zimmerman98 or oil palmReference Danielsen, Beukema, Burgess, Parish, Bruhl, Donald, Murdiyarso, Phalan, Reijnders, Struebig and Fitzherbert99. These transitions from diverse perennial to simplified monocultural systems are often accompanied by negative consequences for hydrologic servicesReference Martinez, Perez-Maqueo, Vazquez, Castillo-Campos, Garcia-Franco, Mehltreter, Equihua and Landgrave100. Notably, this example underscores the interlinked nature of biodiversity and perenniality: shade-grown coffee systems are typically managed for multiple products in addition to coffee, many for local consumption (e.g., fruits, herbs and medicinals), whereas a transition to sun-coffee generally involves more intensive management of a single product—coffee. Although difficult to disentangle, especially given the lack of controlled studies, it is likely that many of the ES that are enhanced by perennial systems are inextricably linked with the concomitant increase in biodiversity.
In contrast to humid climatic regions, in arid environments the extraction of too much water by perennials can be problematic for water supply, as water use by forests and shrublands is almost always greater than by croplands or grasslandsReference Zhang, Kang and Zhang101 and planting trees typically reduces annual water yieldReference Farley, Jobbagy and Jackson102. In China, where large-scale revegetation initiatives in arid regions have resulted in the planting of millions of hectares of treesReference Yang, Zhang, Jia and Ci103, a recent study found that the total water loss due to transpiration from a poplar plantation was 100% greater than that of adjacent shrublandReference Wilske, Lu, Wei, Chen, Zha, Liu, Xu, Noormets, Huang, Wei, Chen, Zhang, Ni, Sun, Guo, McNulty, John, Han, Lin and Chen104. Controversy over tree planting and water supply is especially acute concerning exotic species, with much of the debate focusing on fast-growing eucalyptus and pine speciesReference Casson105. Crucial to determining the overall cost–benefit relationship of planting trees in drylands will be quantifying the net trade-offs between increased transpiration, on the one hand, and improved infiltration and water storage capacity and reduced runoff on the otherReference Illstedt, Malmer, Elke and Murdiyarso106. Notably, trees in semi-arid regions may provide many ES that outweigh their increased water use, including fuelwood, timber poles, nutritional products and windbreaksReference Davidson107, improving soil properties such as porosityReference Oyedele, Awotoye and Popoola108, infiltrationReference Hansson109, Reference Torquebiau and Kwesiga110, water storage capacityReference Gebresamuel, Singh and Dick111, and nutrient cycling and soil fertilityReference Rao, Mafongoya, Kwesiga and Maghembe112. Trees may also increase water content in drylands by ameliorating microclimate and reducing soil evaporationReference Sanou, Zougmore, Bayala and Teklehaimonot113, Reference Kho, Yacouba, Yaye, Katkore, Moussa, Iktam and Mayaki114, or by hydraulic lift or redistributionReference Bayala, Heng, van Noordwijk and Ouedraogo115–Reference Ludwig, Dawson, de Kroon, Berendse and Prins117.
In semi-arid areas of Africa, the positive effects of trees in cropping systems are exemplified by farmers’ use of Faidherbia (Acacia) albida, a leguminous tree with a reverse phenological pattern relative to most other trees (e.g., leaves drop in the wet season and flush in the dry season), which may reduce competition for resources with crops during the growing season. Crop yields in grain fields with interplanted F. albida trees have increased by over 200%, even without additional fertilizer inputs, while these mixed tree–food crop systems also provide multiple benefits such as fuelwood, fodder and enhanced food security during severe droughtReference Garrity, Akinnifesi, Ajayi, Weldesemayat, Mowo, Kalinganire, Larwanou and Bayala118, Reference Syampungani, Chirwa, Akinnifesi and Ajayi119. Similarly, in dry temperate climate regions in China, widespread incorporation of perennial plants into annual row crops is occurringReference Qiao, Chen, Hong, Li and Liu120, Reference Zhao, Shanm, Deng, Zhao, Zhang and Wang121, even though most of these lands do not have irrigation. These perennial plantings are being used to reduce sediment and pollutant exportReference Wang, Yin and Shan122, stabilize dunesReference Qiao, Chen, Hong, Li and Liu120, conserve soil water and nutrientsReference Guo, Huang, Jiang and Lv123, enhance N-fixationReference Xin124 and supplement local economic incomesReference Wilske, Lu, Wei, Chen, Zha, Liu, Xu, Noormets, Huang, Wei, Chen, Zhang, Ni, Sun, Guo, McNulty, John, Han, Lin and Chen104, Reference Bao, Wu and Zhang125. Another positive effect of planting deep-rooted perennials in some drylands, such as southern Australia and the African Sahel, is to alleviate problems of salinization of soils and groundwater by lowering water tablesReference Scanlon, Jolly, Sophocleous and Zhang43, Reference Gordon, Peterson and Bennett126. Studies have also demonstrated the influence of different typesReference Pan, Ma and Shangguan127, Reference Wang, Yin and Shan128 and speciesReference Dass, Sudhishri, Lenka and Patnaik129, Reference Vigiak, Ribolzi, Pierret, Sengtaheuanghoung and Valentin130 of perennial vegetation on enhancing hydrologic functions and reducing sediment and nutrient losses in these regions; however, relatively little work has specifically examined the importance of landscape position.
The impacts of incorporating perennial vegetation within cropping systems on hydrologic services are clearly being documented across different regions and agroecosystems, and these studies are highlighting the importance of considering both the local climate–vegetation interactions and management objectives when selecting suitable perennialzation approaches. Opportunities for significant hydrologic benefits through perennialization may be particularly strong in humid regions where enhanced hydrologic regulation is a priority, while significant ES gains are also being achieved in dryland regions through strategic placement of small amounts of perennial vegetation. More work is required to better understand how varying the spatial location, type and amount of perennial vegetation can differentially influence hydrologic outcomes, especially at the watershed scale, in order to maximize benefits under diverse climatic conditions.
Climate Regulation and Climate Change Mitigation and Adaptation
Although agroecosystems can provide important climate regulation services through carbon (C) sequestration and storage, they can also increase greenhouse gas emissions as a consequence of biomass burning or accelerated decomposition during forest conversion processes, as well as from chemical and energy inputsReference Canadell, Le Quere, Raupach, Field, Buitenhuis, Ciais, Conway, Gillett, Houghton and Marland131, Reference Grace, Robertson, Millar, Colunga-Garcia, Basso, Gage and Hoben132. Whether an agroecosystem is a net carbon sink or source is directly influenced by management practices and their interaction with local biophysical and climatic conditionsReference Bailey, Motavalli, Udawatta and Nelson133. As diverse perennial plant communities typically store more carbon than annual or short-rotation cropping systemsReference Zan, Fyles, Girouard and Samson10, Reference Tolbert, Todd, Mann, Jawdy, Mays, Malik, Bandaranayake, Houston, Tyler and Pettry134, perennialization provides an opportunity for mitigating climate change while sustaining agricultural productivity, especially when targeting those species combinations and landscape positions that maximize potential carbon sequestration benefits.
In the Midwestern US, the native tallgrass prairie ecosystem historically stored large amounts of carbon belowground relative to the annual cropping systems they replaced. Conversion of prairie to agriculture resulted in an estimated loss of 24–89% of the original soil organic C and total NReference Jelinski and Kucharik135–Reference West and Post138. Estimated annual accumulation rates of soil organic C following reestablishment of perennial grasslands on former agricultural lands vary greatly, from 19.7 to 78 g C m−2 yr−1 in the upper 10 cm soil horizonReference Knops and Tilman136, Reference Baer, Rice and Blair139–Reference McLauchlan142, with average reported rates around 30 g C m−2 yr−2Reference Post and Kwon143, Reference Schlesinger144. However, rebuilding soil organic pools to pre-agriculture levels will require time. For the Great Plains region, McLauchlanReference McLauchlan142 estimated that reestablishment of perennial grasslands on former agricultural lands could rebuild soil organic C pools to levels equivalent to unplowed native prairie within 55–75 years, while Matamala et al.Reference Matamala, Jastrow, Miller and Garten145 estimated that within a century restored tallgrass prairie vegetation in Illinois would reach 50% of its soil C storage potential. Differences in C accrual rates across sites and soil depths due to variation in root distribution, rhizosphere activityReference Culman, DuPont, Glover, Buckley, Fick, Ferris and Crews146, Reference Wilson, Barnes, Koen, Ghosh and King147 and vertical retranslocation of soil CReference David, McLsaac, Darmody and Omonode47, Reference Culman, DuPont, Glover, Buckley, Fick, Ferris and Crews146 may account for some of this variability.
Notably, C storage benefits are quickly lost when perennial vegetation reverts to annual crops, leading to rapid releases of CO2 and other greenhouse gases to the atmosphereReference Grandy and Robertson140, Reference Sequin, Arrouays, Balesdent, Soussana, Bondeau, Smith, Zaehle, de Noblet and Viovy148. Gelfand et al.Reference Gelfand, Zenone, Jasrotia, Chen, Hamilton and Robertson149 examined the greenhouse gas consequences of converting land under the Conservation Reserve Program (CRP) to continuous corn, corn–soybean or perennial grass for biofuel production. They found that projected C debt repayment periods under no-till management ranged from 29 to 40 years for corn–soybean and continuous corn, respectively, whereas the use of existing CRP grasslands for cellulosic feedstock production would avoid C debt entirely and provide modest climate change mitigation immediately.
Landscape position can also influence the rate of C sequestration and storage potential of perennial vegetation. Some studies suggest that C sequestration potential may be maximized by placing perennial cover in topographical positions with higher moisture availability, such as footslopes and riparian areasReference Jelinski and Kucharik135, Reference Weaver150. On the other hand, because cultivation of sensitive areas such as shoulder and backslope positions may accelerate erosion of soil organic matterReference Aguilar, Kelly and Heil151, Reference Pennock and van Kessel152, targeting topographical positions that are vulnerable to erosion can promote C retention in the soil. More work is needed to refine understanding of the site-specific features that influence the relationship between topographic position and C sequestration.
Carbon sequestration potential in soils and plant biomass may also vary depending on both species identityReference De Deyn, Quirk, Yi, Oakley, Ostle and Bardgett153 and functional compositionReference Fornara and Tilman154 of the vegetation. In general, trees and shrubs sequester and store greater amounts of C compared with herbaceous plants due to their larger total above- and below-ground biomassReference Peichl, Thevathasan, Gordon, Huss and Abohassan155. In the Midwestern US, riparian buffer systems consisting of mixtures of native herbaceous and woody species sequestered significantly greater amounts of C relative to crop fields or cool-season grass buffersReference Tufekcioglu, Raich, Isenhart and Schultz54. Nevertheless, grassland vegetation can be highly effective at accumulating C by depositing large amounts of organic matter deeper in the soil horizon where it is protected from decompositionReference Matamala, Jastrow, Miller and Garten145, Reference Bashkin and Binkley156. Conversely, woody plants typically deposit a large fraction of total organic matter inputs on the ground surface, where decomposition occurs more rapidlyReference Post and Kwon143. Knowledge of how different species and functional groups affect rates of C sequestration, patterns of partitioning of C between above- and belowground biomass, and soil organic C accrual and depletion rates is important to take into account when using perennial vegetation as part of climate change mitigation strategiesReference Laungani and Knops157.
Not only do perennials store additional C relative to annual or simplified cropping systems, perennial plant communities may also enhance agroecosystem resilience and stability to environmental fluctuations, especially when they include high levels of biodiversityReference Lin22–Reference Tilman, Hill and Lehman24, Reference Wolters, Silver, Bignell, Coleman, Lavelle, Van der Putten, de Ruiter, Rusek, Wall, Wardle, Brussaard, Dangerfield, Brown, Giller, Hooper, Sala, Tiedje and van Veen158. In the Midwestern USA, conservation agriculture practices are increasingly promoting the use of diverse mixtures of native tallgrass prairie species rather than plantings of a single native or introduced species typically used in the past (e.g., Bromus inermis, brome; Bouteloua cuttipendula, side oats; and Panicum virgatum, switchgrass).
Research suggests that grass and forb species typical to the native tallgrass prairie possess a variety of ecophysiological traits and adaptive strategies that enhance resilience and resistance to climatic extremes, such as high water use efficiencyReference Knapp159–Reference Nippert, Fay, Carlisle, Knapp and Smith161, the capacity to senesce and resproutReference Novoplansky and Goldberg162, Reference Xu and Baldocchi163, deep rootsReference Tufekcioglu, Raich, Isenhart and Schultz164, Reference Weaver165 and high ecological plasticityReference Asbjornsen, Mora and Helmers166, Reference Hayes and Seastedt167. In addition, greater vertical and horizontal heterogeneity in more diverse plant communities allows for niche partitioning and complementary use of resourcesReference Nippert and Knapp168, which on the landscape scale can help buffer ecosystems against extreme climate fluctuationsReference van Nes and Scheffer169. In contrast, annual crops in temperate humid climates are particularly vulnerable to extreme weather conditions, due to a combination of shallow rooting, a relatively short (3–4 months) growth period, high water demand, and high sensitivity to drought and heat stressReference Benjamin and Nielsen170–Reference Traore, Carlson, Pilcher and Rice174. Perennial plant communities with high species diversity may also have a greater probability of containing species with traits adapted to environmental changes, thus conferring greater ‘insurance’ relative to plant communities with low species diversity or monoculturesReference Hooper and Vitousek175, Reference Naeem and Li176. Some experimental support for enhanced resilience in diverse perennial plant communities in the context of climate change is emergingReference De Boeck, Lemmens, Bossuyt, Malchair, Carnol, Merckx, Nijs and Ceulemans177–Reference Van Peer, Nijs, Reheul and De Cauwer180, especially in terms of greater temporal stabilityReference Tilman, Hill and Lehman24. Nonetheless, the relationship between biodiversity and the sustainability of ES is complexReference Lin, Perfecto and Vandermeer27, Reference Swift, Izac and van Noordwijk181, and more work is needed to better design integrated annual–perennial landscapes capable of adapting to future climate change while sustaining diverse ES.
In the humid tropics, conversion of forests and traditional agroforestry systems to more intensive agricultural systems has contributed substantially to global greenhouse gas emissions and changes in C stocksReference DeFries and Rosenzweig182, Reference Gibbs, Brown, Niles and Foley183. In Central America and Mexico, agroforestry systems that maintain a significant tree component, such as shade coffee and cacao plantations, home gardens and tree farms, are particularly effective at sequestering and storing C, while providing diverse agricultural productsReference Segura, Kanninen and Suarez184, Reference Soto-Pinto, Anzueto, Mendoza, Ferrer and de Jong185. Kirby and PotvinReference Kirby and Potvin186 found that while native forests in Panama managed with selective logging stored more than twice as much C than forests managed under traditional agroforestry practices, agroforests stored three times more C than pasture. Expanding agroforests into pasture could provide additional biodiversity and livelihood benefits that the most common reforestation system in the region—monoculture teak plantations—does not provide. A meta-analysis examining the potential for soil organic C accumulation through afforestation found that planting trees on croplands resulted in more pronounced increases in soil organic C stocks relative to pastures or grasslands, and that broadleaved tree species had a greater capacity to accumulate soil organic C than coniferous speciesReference Laganiere, Angers and Pare187.
In dry climatic regions where plant growth is relatively slow and total plant biomass is low, the potential for sequestering and storing C per unit land area is generally less than in moist climatic regions. However, the total area of degraded drylands that could potentially be restored to perennial vegetation cover is so large that it constitutes a significant potential C sinkReference Berndes188. Studies in dryland regions of Africa have shown that the amount of soil organic matter below tree canopies is significantly greater compared with open areas due to high inputs of leaf litter and higher root massReference Bayala, Balesdent, Marol, Zapata, Teklehaimanot and Ouedraogo189–Reference Takimoto, Nair and Nair191. In the case of evergreen agricultural systems in semi-arid Africa (discussed previously), incorporating Faidherbia albida trees has been shown to accumulate between 2–4 Mg ha−1 yr−1 of above- and below-ground C, which represents a 10-fold increase over conventional conservation farming systemsReference Kaonga and Bayliss-Smith192–Reference Makumba, Akinnifesi, Janssen and Oenema194. Targeting of woody perennials in dryland regions for enhanced C storage should focus on the preservation or introduction of widely dispersed trees within agroecosystems in order to maximize total ES benefits, while maintaining a favorable balance between carbon–water trade-offs.
In semi-arid regions of Africa, the traditional agroforestry parklands are also used by local farmers to adapt and reduce vulnerability to climatic fluctuations and other extreme weather conditions. These parklands typically comprise several species and genera that constitute important sources of medicine, fruits, oils, leaves, nuts and spices that are main components of the local dietReference Bayala, Heng, van Noordwijk and Ouedraogo115, Reference Bayala, Teklehaimanot and Ouedraogo195–Reference Jonsson, Ong and Odongos197, while also providing fuelwood, feed and an additional income sourceReference Kumar and Nair91. Similarly, shade coffee agroforestry systems in the humid tropics have been shown to provide better protection for crop plants from extremes in microclimate and soil moisture relative to low-shade (10–30%) systems, thus representing an important adaptive strategy for farmers to future climatic extremesReference Lin198. As woody trees and shrubs are often better able to survive extreme weather events such as droughts or floods, they are particularly important for meeting the nutritional and health needs of local people during times of food scarcity.
Although the potential for C sequestration in agricultural soils worldwide has been estimated to be relatively modest, representing approximately 3–6% of fossil fuel C emissions, practices that enhance C storage within agricultural landscapes are considered an important component of climate change mitigation strategiesReference Hutchinson, Campbell and Desjardins199. Globally, a meta-analysis by Ogle et al.Reference Ogle, Breidt and Paustian200 found that soil organic C was most sensitive to management impacts in tropical moist regions, followed by tropical dry, temperate moist and, lastly, temperate dry regions. Further, the tropics lose nearly two times as much C and produce less than one-half the annual crop yield compared with temperate regionsReference West, Gibbs, Monfreda, Wagner, Barford, Carpenter and Foley201. This situation underscores the importance of avoiding clearing more tropical forests for agriculture while balancing agricultural intensification with targeted opportunities for C sequestration through perennialization of existing agricultural landsReference DeFries and Rosenzweig182. Another promising approach to promoting greater C sequestration on agricultural lands in both temperate and tropical regions is the use of perennial plants as biofeedstock for cellulosic ethanol production (potentially reducing the use of fossil fuels) combined with application of the charcoal by-product—the biochar—to agricultural soils to increase C storage over long time periods, given that biochar is highly recalcitrant to degradation (see the ‘Provisioning Services’ section for a more detailed discussion)Reference Laird202, Reference Atkinson, Fitzgerald and Hipps203.
Biotic Regulation
Perennialization can increase landscape complexity and resource heterogeneity, thereby promoting habitats for diverse communities of beneficial organisms that help control pests and pathogens, and provide pollination services in adjacent crop fieldsReference Fiedler, Landis and Wratten204. Perennials also contribute to food web services by providing habitat for organisms that are a food source for auxiliary biota in the productive or semi-natural sub-systems, and for biota that feed on crop pestsReference Pluess, Opatovsky, Gavish-Regev, Lubin and Schmidt-Entling205.
As perennial vegetation is generally subject to lower levels of disturbance and is more stable over time than are ephemeral annual crop systems (with some exceptions, such as severe over-grazing and hay and grass silage production systems subjected to frequent harvests), various forms of perennial cover can provide critical elements of ‘conservation biological control’ strategies that seek to retain and facilitate populations of predators and parasitoids that attack arthropod pests in adjacent croplandReference Bianchi, Booij and Tscharntke206–Reference Landis, Wratten and Gurr208. Both within-field and around-field perennial vegetation can maintain and increase the impacts of natural enemies of crop pests through the provision of alternative prey species, pollen and nectar used as protein and energy sources, and habitat for over-wintering and reproductionReference Fiedler and Landis11, Reference Gurr, Wratten and Luna209–Reference Woltz, Isaacs and Landis212. Within-field diversification with perennial plants can be set up directly by individual farmers, whereas around-field diversification with perennials can reflect the cumulative impacts of multiple landowners and land managers on landscape structure.
A number of studies indicate that increasing the proportion of landscapes occupied by woodlots, hedgerows, old-field fallows and pastures can enhance biological control of crop pests in fields embedded within those forms of perennial vegetationReference Bianchi, van Wingerden, Griffioen, van der Veen, van der Straten, Wegman and Meeuwsen213, Reference Thies and Tscharntke214. Tscharntke et al.Reference Tscharntke, Steffan-Dewenter, Kruess and Thies215 reported that parasitism of rape pollen beetles (Meligethes aeneus F.) was greater in German landscapes in which non-crop vegetation occupied >20% of total land area compared with landscapes in which non-crop vegetation occupied <20% of the land area. In the less diverse landscapes, parasitism of rape pollen beetles was greater at the edges of fields than in the centers; in contrast, in the more vegetatively diverse landscapes, parasitism rates did not differ between field edges and centers. Scale issues were addressed in a study conducted by Gardiner et al.Reference Gardiner, Landis, Gratton, DiFonzo, O'Neal, Chacon, Wayo, Schmidt, Mueller and Heimpel216 at sites within four states in the north central US. Densities of predatory coccinellid beetles and rates of biological control of soybean aphid (Aphid glycines) increased as the proportion of landscapes occupied by forest and grassland rose, and the proportion occupied by corn and soybean dropped, with diversity and composition evaluated at a scale of 1.5 km around focal fields explaining the greatest proportion of variation in coccinellid densities and biological control rates. Thus, for certain host-specific crop pests and their natural enemies, there is likely to be a need for coordinated management activities in establishing and maintaining perennial vegetation at the landscape scale.
Similarly, perennial vegetation can provide a higher abundance and greater diversity of refuges for pollinator populations that are essential for many fruit and vegetable crops. In agriculture, especially among pollen-limited crops, promoting pollination services is an important means of increasing productivity. In a review of pollination services for the leading food crops in 200 countries, Klein et al.Reference Klein, Cunningham, Bos and Steffan-Dewenter217 found that of a total of 107 crops traded on the world market that are directly consumed by humans (but excluding crops that are solely passively self-pollinated, wind-pollinated or parthenocarpic), 40% of these crops are highly to moderately dependent on animal pollinators. Significant declines in different pollinator species have been documented in the Midwestern US, raising several ecological and economic concernsReference Grixti, Wong, Cameron and Favret218–Reference Hendrix, Kwaiser and Heard220. Grixti et al.Reference Grixti, Wong, Cameron and Favret218 noted that half of the bumblebee species found historically in Illinois have been locally extirpated or have suffered declines, supporting observations of broader declines in North America. Major declines in the bumblebee fauna coincided with large-scale agricultural intensification. Bee richness was positively related to plant richness and abundance of potential nesting resources, and bee community composition was significantly related to plant richness, soil characteristics potentially related to nesting suitability, and canopy coverReference Grundel, Jean, Frohnapple, Glowacki, Scott and Pavlovic219. Conservation of agriculturally important pollinators may be promoted by applying wildlife-friendly approaches to agriculture, such as increasing agricultural land set-asides and hedgerowsReference Hendrix, Kwaiser and Heard220.
In humid temperate regions, perennial cover crops growing beneath ‘main’ crops have been shown to contribute to biological control, but they can also reduce crop yields through competition for water and nutrients, making their use problematic in many situationsReference Bugg221–Reference Schmidt, O'Neal and Singer224. An alternative form of within-field diversification involves the use of narrow strips of perennial vegetation that provide habitat for predators and parasitoids of pests in closely adjacent cropped areas. This approach has been studied in wheat fields where perennial grasses function as ‘beetle banks,’ conserving coleopteran predators that forage on cereal aphidsReference Collins, Boatman, Wilcox, Holland and Chaney225, Reference MacLeod, Wratten, Sotherton and Thomas226, and in vineyards where strips of flowering plants provide nectar and pollen sources and a corridor from riparian areas for predators attacking thrips and leafhoppersReference Nicholls, Parrella and Altieri227. Strips of alfalfa (Medicago sativa) have been shown to conserve predatory beetles, bugs, lacewings and spiders, and increase their densities in adjacent strips of cotton, thereby exerting greater control of mirid and lepidopteran pestsReference Mensah, Sequeira, Gurr, Wratten and Altieri228. Strips of perennial grasses, legumes and other forbs left unsprayed with pesticides formed refuges for predatory ground beetles, permitting rapid recolonization of chemically disturbed cornReference Lee, Menalled and Landis229.
Compared with arthropod pests and their natural enemies, the effects of perennial vegetation on crop diseases have received much less attention from the scientific community. In artificially constructed assemblages of perennial prairie species, increases in plant species diversity reduced the severity of diseases caused by wind- and rain-dispersed pathogens on four target plant species (Septoria liatridis on Liatris aspera, Uromyces lespedezae-procumbentis on Lespedeza capitata, Ersiphe cichoracearum on Monarda fistulosa, and Colletotrichum spp. on Schizachyrium scoparium)Reference Knops, Tilman, Haddad, Naeem, Mitchell, Haarstad, Ritchie, Howe, Reich, Siemann and Groth230, suggesting that diverse mixtures of perennial species interplanted with crops might offer protection against certain pathogens. Such protection could occur as the result of non-crop vegetation acting as a barrier against propagule dispersal, hosting antagonistic and competitive microbes, altering microclimate conditions or inducing resistance within cropsReference Finckh, Wolfe, Cooke, Jones and Kaye231. Perennial grain crops may also be bred for high levels of disease resistance. Hayes et al.Reference Hayes, Newell, DeHaan, Murphy, Crane, Norton, Wade, Newberry, Fahim, Jones, Cox and Larkin232 noted that wheatgrass species (Thinopyrum and Agropyron spp.) conveyed resistance to many diseases when crossed with wheat to produce perennial hybrids.
Cox et al.Reference Cox, Garrett and Bockus233 noted that typical cultural practices for reducing soil- and residue-borne pathogens, such as annual crop rotations, delayed planting dates and tillage, are not feasible in perennial crop systems, and that as a consequence, pathogens that survive in soil, crop residues, live shoots and roots may become an increasing threat to perennial crops and adjacent areas of susceptible annual crops over time. To impede the build-up of pathogen populations, Cox et al.Reference Cox, Garrett and Bockus233 suggested three approaches: planting mixtures of perennial crop cultivars or species that vary in disease resistance to particular species and strains of pathogens; using grazing to remove plant residues; and burning plant residues, a natural phenomenon in perennial grass systems that can decrease the incidence and severity of a number of diseases. These methods remain to be field-tested in perennial crop and mixed perennial–annual crop associations, however.
The pest problem is especially important in the humid tropics, where the high rainfall and low seasonality favor two key groups of natural plant enemies—insects and fungiReference Givnish234. Consequently, the potential for losing crops to disease is far greater than in more temperate climates. Conserving both the associated fauna within tropical agroforests and surrounding forest fragments with high quality agricultural matrices is critical to maintaining pest regulation servicesReference Philpott and Armbrecht235. For example, levels of ant biodiversity exhibit sharp declines with the intensification of coffee and cacao production systems compared with traditional agroforestry systemsReference Philpott, Perfecto and Vandermeer236. High diversity and abundance of ants provide important ES in coffee agroecosystems since ants are an important predator groupReference Philpott and Armbrecht235, Reference De la Mora, Livingston and Philpott237. Another predator studied in the coffee agroforest, birds, helps control coffee berry borers and thus increases coffee yield and farm income, a potentially important conservation incentive for producersReference Johnson, Kellermann and Stercho238, Reference Kellermann, Johnson, Stercho and Hackett239. Landscape heterogeneity may allow mobile predators to provide pest control broadly, despite localized variation in farming intensities. Similarly, forest remnants provide nearby coffee plantations with a diversity of bees and other pollinators that can increase both the amount and stability of pollination services by reducing dependence on a single introduced pollinatorReference Brosi, Daily, Shih, Oviedo and Duran240–Reference Ricketts242, and improve both quantity and quality of coffee yields by reducing the frequency of misshapen seedsReference Ricketts242.
Recent studies in the dry tropical zone have documented the importance of hedgerows (often planted for soil conservation purposes along sloping land) for enhancing biological pest control by serving as refugia for predators. Girma et al.Reference Girma, Rao and Sithanantham243 found that corn associated with hedgerows comprised of nine different species experienced significantly lower stalk borer (Busseola fusca and Chilo spp.) and aphid (Rhophalosiphum maidis) infestations than pure stands of corn. The study also found, however, that hedgerow effects on pest infestations of crops cannot be generalized but depend on the specific arthropods and their refugia preferencesReference Girma, Rao and Sithanantham243.
Trees have been shown to affect pest infestations in dry tropical agroecosystems by acting as barriers to movement of insects, masking the odors emitted by other components of the system, and sheltering herbivores and natural enemiesReference Rao, Singh and Day244. In sub-Saharan Africa, where the corn stalk borer, Busseola fusca (Full) (Noctuidae), and the sorghum stem borer, Chilo partellus Swinh (Pyralidae), pose major threats to food production, Khan et al.Reference Khan, Pickett, van den Berg, Wadhams and Woodcock245, Reference Khan, Midega, Hutter, Wilkins and Wadhams246 reported positive effects of trap crop plants (Napier grass, Pennisetum purpureum, and Sudan grass, Sorghum sudanensis) and intercrop plants (molasses grass, Melinis minutiflora, and Desmodium spp.) for controlling stem borer outbreaks through release of attractant and repellent semiochemicals.
Currently, it is difficult to view perennial vegetation as a panacea for crop pest and disease problems, though promising opportunities for improved management strategies clearly exist. The generally positive influence of perennial vegetation on biological control is not necessarily consistent from year to yearReference Menalled, Costamagna, Marino and Landis247. Moreover, in some cases perennial vegetation can exacerbate pest problems by serving as an alternate host to crop pests, as is the case for soybean aphid, which resides on buckthorn (Rhamnus spp.) growing in hedgerows and woodlands adjacent to soybean fields in the Midwestern USAReference Ragsdale, Voegtlin and O'Neil248. Much more needs to be learned concerning sources of spatial and temporal variability in the effects of perennial vegetation on natural enemies of crop pests and crop disease dynamics, and a much more detailed understanding needs to be developed concerning how individual perennial species affect natural enemies of arthropod pests, crop pathogens and crop yields.
Soil Quality and Nutrient Cycling
Soil quality is the capacity of a soil to sustain biological productivity, maintain environmental quality, and promote plant and animal healthReference Doran, Parkin, Doran, Coleman, Bezdicek and Stewart249, Reference Karlen, Mausbach, Doran, Cline, Harris and Schuman250. Consequently, soil quality is a required supporting service for agroecosystems and human societies. Over the past several decades, soil scientists have assembled sets of physical, chemical and biological indicators of soil qualityReference Doran, Parkin, Doran, Coleman, Bezdicek and Stewart249. Physical indicators include rates of water flow into and through soil; soil structural characteristics, such as porosity, bulk density and aggregate size distribution; and measures of stability, such as aggregate stability in water and erosion rate. Chemical indicators include quantities and qualities of soil organic C and N, nutrient availability to plants and soil reaction (pH). Biological indicators include the mass of organisms in soil and measures of their physiological activity, including rates of respiration and enzymatic reactions.
Regular additions of organic matter, especially root materials, play a critical role in the maintenance of soil qualityReference Russell, Cambardella, Laird, Jaynes and Meek251, Reference Weil, Magdoff, Magdoff and Weil252. In general, in humid, temperate environments, woody and herbaceous perennial plants add more root material and organic matter to soil than do annual cropsReference Liebig, Johnson, Hanson and Frank53, Reference Tufekcioglu, Raich, Isenhart and Schultz54 and consequently they are more effective at maintaining soil quality. As noted in the previous sections on hydrologic regulation and climate regulation, rainfall infiltration, hydraulic conductivity and soil C storage are greater for soil occupied by trees and herbaceous perennials than annual row crops.
Adding perennial crops to rotation sequences dominated by annual crops can have beneficial effects on soil quality and function. Residues of alfalfa, red clover and other perennial forage legumes can release enough N to satisfy much or all of the nitrogen demand of subsequent cereal crops, such as corn nitrogenReference Fox and Piekielek253, Reference Morris, Blackmer and Elhout254. Russell et al.Reference Russell, Laird and Mallarino255 compared cropping systems based on annual crops (continuous corn or a corn–soybean rotation) with systems that contained alfalfa as well as corn and soybean and found that systems containing alfalfa had greater available N, higher rates of potential net N mineralization, and more microbial biomass C. Soil total N and organic C concentrations were also higher in systems containing alfalfa. Thus, cropping systems that contain perennial legumes in sequence with annual crops can have lower requirements for fertilizer and higher rates of soil C storageReference Gregorich, Drury and Baldock256, Reference Gregorich, Rochette, VandenBygaart and Angers257.
More efficient N use by perennials can potentially reduce the environmental impacts associated with nutrient losses. Nitrogen losses to drainage water are greater for corn and soybean than for perennial grasses and herbaceous legumes such as alfalfaReference Randall, Huggins, Russelle, Fuchs, Nelson and Anderson258. Moreover, trees can have even higher N and P removal efficiency than perennial grassesReference Tufekcioglu, Raich, Isenhart and Schultz54, Reference Zhang, Liu, Zhang, Dahlgren and Eitzel67. In Iowa, N immobilization rates in poplar stands and switchgrass sites averaged 37 and 16 kg N ha−1 yr−1, respectivelyReference Tufekcioglu, Raich, Isenhart and Schultz54.
For humid tropical regions, which typically have soils of low fertilityReference Richards, Walsh, Baillie and Greg-Smith259, it is especially important to develop land-use systems that promote efficient nutrient cyclingReference Schroth, Elias, Uguen, Seixas and Zech260. Land-use systems based on tree crops (e.g., multistrata agroforestry systems) have clear advantages over annual cropping systems for the maintenance of soil fertility because they provide permanent soil cover and root systemsReference Schroth, Elias, Uguen, Seixas and Zech260. For example, when shade coffee agroforestry systems are converted to more intensive sun-grown systems by removing the shade trees, N fertilizers are usually applied at high rates to maintain productivity. Research assessing the consequences of these changes for nitrate leaching and water quality has yielded contrasting resultsReference Babbar and Zak261–Reference Harmand, Avila, Dambrine, Skiba, de Miguel, Renderos, Oliver, Jimenez and Beer263. Babbar and ZakReference Babbar and Zak261 reported lower N leaching rates from shaded than from unshaded coffee cultivation in Costa Rica (9 versus 24 kg ha−1 yr−1, respectively), though plantations differed in the density and pruning regime of the coffee plants, making the data difficult to interpret. Harmand et al.Reference Harmand, Avila, Dambrine, Skiba, de Miguel, Renderos, Oliver, Jimenez and Beer263 found that drainage was slightly reduced in a shaded compared with an unshaded coffee plantation, but N losses in surface runoff were low in both cases. In general, shade coffee systems appear to have greater potential to enhance other soil fertility properties such as nutrient and organic inputs from litterfallReference Beer, Muschler, Kass and Somarriba262, Reference Jaramillo-Botero, Santos, Fardim, Pontes and Sarmiento264 and earthworm densityReference Leon, De Melo, Soto, Johnson-Maynard and Lugo-Perez265 than their unshaded counterparts.
In arid agricultural regions of East Africa, retaining savanna trees as part of annual cropping systems can enhance soil fertilityReference Belsky266 and, in turn, understory crop productionReference Ong and Leakey267, Reference Van Noordwijk and Ong268. Perennialization can also help mitigate or even reverse processes of soil loss and desertification, common in many drylands, by reducing the movement of sand, retaining soil organic matter and promoting establishment of new vegetation. For example, in the Loess Plateau region of China, Qiao et al.Reference Qiao, Chen, Hong, Li and Liu120 demonstrated that mixed tree plantations interspersed within cropland can, over time, help stabilize and facilitate the restoration of native vegetation. Yuan et al.Reference Yuan, Bingner and Locke269 studied the effects of mixing wheat with three perennials (Stipa bungeana, Lespedeza davurica and Artemesia capillaries) in the same region, and found that the perennials significantly increased the total crop production, especially root growth.
Overall, the influence of perennials on soil fertility is synergistically coupled with their impact on hydrologic and C sequestration services, and thus, multiple ES can be obtained through perennialization processes in cropland. However, as with hydrological and C sequestration services, more work is needed to improve understanding of how different species mixtures, amounts and landscape positions influence soil quality properties, in both temperate and humid agroecosystems.
Provisioning Services
Food, fiber and fuel production to meet subsistence or market needs is the overwhelmingly dominant goal of agriculture. Crops in individual fields are dependent on services provided by nearby ecosystems, whether native or managed, and nearby ecosystems are often influenced by their agricultural neighborsReference Swinton, Lupi, Robertson and Hamilton7. As discussed above, perennialization can contribute toward maximizing ES benefits from agricultural landscapes. Below, we review opportunities for accomplishing this goal, with an emphasis on the additional provisioning services that perennial plants also provide.
Forage crops
Following the large-scale introduction in the 1940s and 1950s of relatively inexpensive synthetic fertilizers, farming systems in humid temperate regions have become increasingly specialized, with fewer crops grown within rotation sequences and fewer livestock enterprises integrated with crop enterprisesReference Naylor, Steinfeid, Falcon, Galloways, Smil, Bradford, Alder and Mooney270, Reference Sulc and Tracy271. As a consequence, in regions such as the US Corn Belt, annual grain crops such as corn and soybean have greatly displaced perennial forages, and cattle are often produced in large feedlots, from which manure application to a sufficiently large land base can be problematic. Nonetheless, recent increases in fertilizer and feed costs have led to renewed interest in integrated crop–livestock systems that include perennial species used for pasture and hay, and which exploit the recycling of C and nutrients via manure application to croplandsReference Glover, Cox and Reganold272. Expanding crop sequences based only on annual species to include perennial forages, and returning manure to croplands, can have numerous benefits. As summarized by Russelle et al.Reference Russelle, Entz and Franzluebbers273, these include reduced nitrate leaching to groundwater, reduced rates of erosion by wind and water, increased soil C content and soil water holding capacity, greater biological N fixation and improved nutrient supply, reduced incidence of pests, enhanced grain yield potential, lower fossil energy demands, reduced production costs and improved profitability. Despite these advantages, major disincentives to crop–livestock integration can exist within government farm policies. In the US, for example, disincentives have included subsidies for annual crops, but not perennial forages, and a lack of accounting for costs of the environmental impacts of farming systems. In contrast, Sulc and TracyReference Sulc and Tracy271 noted, ‘… there is significant research investment in and adoption of integrated crop–livestock systems in countries where government price supports for agriculture are limited or nonexistent (e.g., Brazil, New Zealand).’
Grain crops
Although all of the world's current staple grains are annual species, concerns over soil erosion, agrichemical pollution, maintenance of soil organic C stocks and energy costs of tillage have propelled interest among some plant breeders in the development of perennial grain cropsReference Gelfand, Zenone, Jasrotia, Chen, Hamilton and Robertson149, Reference Cox, Bender, Picone, Van Tassel, Holland, Brummer, Zoeller, Paterson and Jackson274. Cox et al.Reference Cox, Bender, Picone, Van Tassel, Holland, Brummer, Zoeller, Paterson and Jackson274 reviewed parental germplasm and methodologies for breeding at least 20 species of perennial grains. Possible approaches to developing perennial grain crops include using related perennial species to add genes into annual crops such as wheat, and domesticating existing perennial species, such as intermediate wheatgrass (Thinopyrum intermedium), Maximilian sunflower (Helianthus maximiliani) and Illinois bundleflower (Desmanthus illinoensis), through selection for higher seed production and desirable agronomic and quality factors. Important biological and technical challenges exist for both approaches, but these might be surmountable in much less time than the millennia that were required to domesticate wheat, maize, rice and other grainsReference Zhou, Helmers, Asbjornsen, Kolka and Tomer71.
Perennial grain crops may have a lower yield potential than annuals due to trade-offs between seed productivity and longevity: resources that might be allocated to seeds may instead have to be allocated to non harvested perennating structures to maintain survival from year to yearReference Cox, Glover, Van Tassel, Cox and DeHaan275. Cox et al.Reference Cox, Glover, Van Tassel, Cox and DeHaan275 argued, however, that perennial grain crops do have the potential to produce high and acceptable seed yields because (1) compared with annual crops, perennials tend to have longer growing seasons, greater canopy cover duration, and deeper rooting depths, allowing them to intercept, retain and utilize more precipitation, nutrients and light; and (2) perennials generally produce more above-ground biomass than do annuals and a greater proportion of that biomass might be reallocated to grain production through breeding. DeHaan et al.Reference DeHaan, Van Tassel and Cox276 and Van Tassel et al.Reference Van Tassel, DeHaan and Cox277 noted that low yields from perennial grain crops to date reflect a lack of attention from plant breeders and they predicted that artificial selection in a properly managed agricultural environment could increase seed yield while maintaining perenniality. In one of the few studies reporting the seed yields of perennial grain crops subjected to artificial selection, Scheinost et al.Reference Scheinost, Lammer, Cai, Murray and Jones278 observed that promising lines of perennial wheat produced 1.7–5.8 Mg ha−1, as compared with 9.0 Mg ha−1 from an annual wheat cultivar commonly grown in the area. Murphy et al.Reference Murphy, Lyon, Balow and Jones279 reported that amphiploid breeding lines derived from crosses between annual wheat and the perennial wheatgrass species Thinopyrum elongatum produced mean grain yields of 0.6–2.2 Mg ha−1, depending on location, which were about 44% of the seed yield of annual wheat grown at the locations. It remains to be learned how much improvement in perennial grain yields can be realized through breeding. Adjustments in soil fertility may be necessary to increase the seed yields of perennial grain species. Loeppky et al.Reference Loeppky, Horton, Bittman, Townley-Smith, Wright and Nuttall280 reported that in northeastern Saskatchewan, seed yields of crested wheatgrass (Agropyron cristatum) and intermediate wheatgrass (A. intermedium) could be increased with the application of N and P fertilizers on soils with low levels of available nutrients. Additional research is needed to better assess the economics of perennial grain production systems, since reduced revenues due to lower yields might be offset by reduced input costs for seeds and land preparation.
Biofuel crops
Growing concerns over the instability of petrochemical prices, heavy dependence on foreign sources of petrochemicals and the environmental impacts of fossil fuel combustion (including greenhouse gas emissions) have led to rapid growth during the past decade in the use of plant materials to produce liquid transportation fuels. In the humid temperate zone, major emphasis has been placed on the use of corn grain to produce ethanol, and soybean and rapeseed to produce biodiesel. The resulting rise in prices of these crops has been viewed favorably by many crop farmers, but has also given rise to pointed critiques on a number of grounds. Partly, but not entirely, because of increased demand from the bioenergy sector for commodity crops, livestock feed costs and food prices for consumers jumped upward in 2006–2007 and may in the future impose financial and nutritional burdens on low-income people in both developed and developing countriesReference Naylor, Liska, Burke, Falcon, Gaskell, Rozelle and Cassman281. Increased crop prices may also spur conversion of land currently in perennial vegetation to arable cropland, with attendant increases in N emissions to water suppliesReference Murphy, Lyon, Balow and Jones279 and greenhouse gas emissions to the atmosphereReference Gelfand, Zenone, Jasrotia, Chen, Hamilton and Robertson149, Reference Fargione, Hill, Tilman, Polasky and Hawthorne282, Reference Searchinger, Heimlich, Houghton, Dong, Elobeid, Fabiosa, Tokgoz, Hayes and Yu283.
Increasing attention to the development of thermochemical and biochemical technologies for converting lignocellulosic materials into liquid fuels and other industrial chemicals adds new twists to the biofuels story. Unlike the currently dominant biofuel technologies that rely on the use of seeds, lignocellulosic conversion processes utilize plant stems and leaves. Consequently, research is being directed to the use of perennial grasses, such as switchgrass (Panicum virgatum) and miscanthus (Miscanthus spp. and interspecific hybrids such as M.×giganteus), and fast-growing tree species, such as poplar (Populus spp.) and willow (Salix spp.), as dedicated bioenergy feedstock cropsReference Adler, del Grosso and Parton284–Reference Volk, Verwijst, Tharakan, Abrahamson and White289. Precedence for the use of dedicated perennial crops as biofuel feedstocks has already been set in Brazil, where sugarcane (Saccharum officinarum) is used for ethanol productionReference Goldemberg290.
Concerns have been raised over the potential for perennial bioenergy crops to become invasive pestsReference Raghu, Anderson, Daehler, Davis, Wiedenmann, Simberloff and Mack291, and protocols should be in place to avert this problem before introductions of non-native species or newly developed genotypes proceed. Tilman et al.Reference Tilman, Hill and Lehman24 proposed the use of diverse mixtures of native prairie plants as bioenergy feedstocks on degraded, low-fertility land, which minimizes risks of introducing noxious species, but also limits the quantities of biomass that might be produced. Further, while removal of large amounts of annual crop biomass can reduce soil fertility and organic C stocksReference Lal292, Reference Wilhelm, Johnson, Hatfield, Voorhees and Linden293, removal of above-ground portions of perennial plants used as bioenergy feedstocks can still allow for the maintenance of soil fertility and organic carbon, since large quantities of nutrients are translocated below ground before shoot harvest, and the more extensive root systems of perennials can sequester sizeable amounts of carbonReference Searchinger, Heimlich, Houghton, Dong, Elobeid, Fabiosa, Tokgoz, Hayes and Yu283, Reference Frank, Berdahl, Hanson, Liebig and Johnson294–Reference Zenone, Chen, Deal, Wilske, Xu, Bhardwaj, Hamilton and Philip296. Residues of biofuel feedstocks can be spread after processing on soil as amendments to return nutrients and CReference Anex, Lynd, Laser, Heggenstaller and Liebman297, Reference Lehmann298.
An important option for the production of perennial biofuel feedstock crops is to use them as one element of multifunctional agricultural landscapesReference Jordan, Boody, Broussard, Glover, Keeney, McCown, McIsaac, Muller, Murray, Neal, Pansing, Turner, Warner and Wyse299. By placing well-adapted perennial biofuel crops on small, but vulnerable portions, of landscapes, soil, water and wildlife conservation benefits might be gained, while providing farmers an additional opportunity for producing a marketable cropReference Schulte, Liebman, Asbjornsen and Crow6, Reference Bhardwaj, Zenone, Jasrotia, Robertson, Chen and Hamilton300. Rotations including perennials and crops, given an appropriate level of management, could be a great opportunity to support the production of biomass and grain while maintaining soil quality over the long term on high-quality agricultural lands. This is especially important in regions with low fertility and where nutrients remain a major constraint, such as in rain-fed farming systems throughout the tropics. Perennials have also been proposed as the major bioenergy plants for the dry temperate zone of China, including short-rotation woody plants and native Achnatherum spp., Agropyron, and other speciesReference Xie, Zhou, Yang and Lu301.
Medicinal products
Another valuable service of perennial plants integrated within agricultural landscapes in many regions worldwide is to provide medicinal products. In China, regardless of Western advances in medical sciences, people rely heavily on medicines derived from combinations of locally produced plants and animals; the majority of medicinal plants are perennialsReference Chen302, Reference Song, Gu and Liu303. In Xingjiang Province alone, for example, 2014 plant species have been used in medical practices, among which 558 species are cultivatedReference Bao, Wu and Zhang125. In the dry tropics of Africa the situation is similar, with a long and rich history of traditional use of perennial plants as medicines, and new phytochemical research has started to link specific plant properties with targeted effects on human healthReference Hall, Tomlinson, Oni, Buchy and Aebischer304, Reference Ouedraogo305. In the wet tropics, there is a long tradition of producing medicinal plants as integral components of home gardensReference Kumar and Nair91.
The above discussion calls attention to the wide range of opportunities that exist for contributing a diverse array of food, fiber and fuel products to human societies through perennial plants. In some cases, these agroecosystems may be dominated by perennial plants, such as in the case of large-scale biofuel production or rotational cropping or grazing systems, but in many situations, they will likely involve the targeted placement of relatively small amounts of perennials in strategic locations as part of a matrix of annual crops. An important focus of future research will be to identify which landscape positions—as well as how much and what type of perennial cover—will provide the greatest benefit in terms of both agricultural products and other diverse ES under contrasting climatic conditions, and to meet different production goals by local communities.
Conclusions
As the global society faces the challenge of meeting increased demand for food, fiber and animal feed to sustain growing populations, while at the same time adapting to a changing climate, agricultural landscapes will likely undergo unprecedented transitions in the near futureReference Jackson, Jobbagy and Nosetto306. Central questions will involve determining the relative value and priority to be given to the many ES provided by agricultural landscapes in addition to food, fiber and feed, and how to best balance these multiple benefits, or in other words, how to harmonize agricultural production and conservationReference Chan and Daily307. In this paper, we used the Corn Belt region of the Midwestern USA as a model system for highlighting examples where scientific research on perennialization of agricultural landscapes has documented progress toward achieving this goal. In particular, we showed how a targeted approach identifies landscape locations and the type and amount of perennial cover for maximizing benefit–cost trade-offs for multiple ES important to society, and thereby providing opportunities for designing multifunctional agroecosystems. We also emphasized that because perenniality and biodiversity are often inextricably and positively linked, and most studies on perennialization to date have not considered their independent effects on enhancing ES, more work in needed to disentangle the relative importance of these two variables. Finally, we highlighted differences in both the constraints and opportunities for perennializing agricultural landscapes among different climate regions globally, in addition to critical gaps in knowledge where more information is needed. Notably, the evidence to date regarding the potential contributions of perennialization to balancing agricultural production and conservation are primarily based on controlled field studies such as those mentioned in this review; the effectiveness of perennialization approaches at the operational scale with farmer participation remains to be tested in the future.
Although an in-depth discussion of the political and socio-economic environment needed to promote perennialization is beyond the scope of the present paper, it is important to acknowledge that effective policy structures and mechanisms, market opportunities, institutional frameworks and infrastructural arrangements will be critical to establish incentives that favor adoption of perennial-based agricultural practicesReference Carpenter and Folke308, Reference Farber, Costanza, Childers, Erickson, Gross, Grove, Hopkinson, Kahn, Pincetl, Troy, Warren and Wilson309. One of the major current constraints to perennialization is that the most economically profitable decision is frequently to grow only a few crop types, and not to invest in conservation of the varieties that are less favored by existing markets. The problem lies both in the public good nature of conservation, and the fact that currently there are no major markets for off-site ES that depend on on-farm agrobiodiversityReference Pascual and Perrings310. Notwithstanding, there are a growing number of initiatives worldwide that seek to create markets for these services, ranging from those based strictly on government intervention to others that are entirely private venturesReference Salzman311, Reference Robertson312. In the USA, the Conservation Reserve Program (CRP) provides monetary incentives to farmers to put cropland into various forms of perennial vegetation, with the original intent of reducing soil erosion. However, this mechanism is vulnerable to fluctuations in land and crop values, as seen by the rapid broad-scale conversion of CRP back to crops in recent years in response to the increasing biofuel economyReference Secchi, Tyndall, Schulte and Asbjornsen313. In the developing world, examples of policies to counter market drivers favoring conversion of traditional, highly diverse perennial-based agroecosystems to more simplified systems include mechanisms such as certificationReference Mas314, Reference Rice and McLean315, payment for ESReference Cole316–Reference Quintero, Wunder and Estrada318 and reducing emissions from deforestationReference Ebeling and Yasue319.
Moreover, it will also be important to quantify the economic implications of perennialization that include the less tangible values of the ES and not only the crops. There are several examples suggesting positive relationships between increasing agroecosystem biodiversity and income for different ES, including pollinator abundanceReference Bugg and Waddington222, Reference Gallai, Salles, Settele and Vaissiére320, pest controlReference Grundel, Jean, Frohnapple, Glowacki, Scott and Pavlovic219, C sequestrationReference Ebeling and Yasue319 and water quality protectionReference Martinez, Perez-Maqueo, Vazquez, Castillo-Campos, Garcia-Franco, Mehltreter, Equihua and Landgrave100, Reference Corbera, Kosoy and Martínez-Tuna321. Gallai et al.Reference Gallai, Salles, Settele and Vaissiére320 estimated that the total economic value of pollination worldwide at $153 billion, which represents 9.5% of the value of the world agricultural production used for human food in 2005. With rare exception, these services are neither prized by markets nor explicitly protected by the law, even though economic studies propose that properly constructed markets would support strong conservation measures2. For public policy decisions to take them into account, non market valuation techniques are urgently neededReference Swinton, Lupi, Robertson and Hamilton7. Economic and social benefits may also be conferred by perennial vegetation through enhanced resilience and stability of agroecosystems, thereby contributing to society's ability to adapt to and cope with climate changeReference Anderies, Ryan and Walker322–Reference Walker, Hollin, Carpenter and Kinzig324.
In order for perennialization practices—and the policies and programs that promote them—to be effective in the long term, scientifically sound data quantifying the effects of perennialization on different ES and identifying specific practices—in terms of what, where and how much perennial cover—that produce maximum benefit–cost trade-offs, will be critical. Our review of the literature suggests that there is substantial scientific evidence suggesting that increasing the presence of perennial vegetation in cropping systems generally leads to greater ES. However, current knowledge about the relationship between the spatial configuration, amount and type of perennial vegetation, and the specific ES impacts is relatively scarce. Further, long-term, replicated studies at the watershed scale, which are critical to addressing the impacts of perennialization on hydrologic services and their relation to other ES, are particularly lacking. Finally, there is generally more information available from temperate climate regions compared with tropical regions. Future work should focus on advancing scientific understanding to address these gaps in order to provide a baseline for designing approaches that enable targeting the placement of appropriate amounts and types of perennial vegetation in strategic locations on the landscape to minimize economic and production costs while enhancing ES.
Acknowledgements
The authors thank the Leopold Center for Sustainable Agriculture, Iowa State University College of Agriculture and Life Sciences, USDA Forest Service Northern Research Station, Iowa Department of Agriculture and Land Stewardship, USDA North Central Region SARE program, USDA AFRI Managed Ecosystems, and the Great Lakes Bioenergy Research Center (GLBRC), for providing funding to support their research activities related to this paper. We also thank Drake Larsen for his assistance with producing the visualizations included in the two figures.