INTRODUCTION
The fan mussel Pinna nobilis (Linnaeus, 1758) is an endemic Mediterranean species probably inhabiting the basin since the lower Miocene (Zunino & Pavia, Reference Zunino and Pavia2009). It is widely distributed in the coastal zone at a depth range of 0.5–60 m (Zavodnik et al., Reference Zavodnik, Hrs-Brenko, Legac, Boudouresque, Avon and Gravez1991) having the anterior part of the shell partially buried in soft substrata, usually in seagrass meadows (Butler et al., Reference Butler, Vicente and de Gaulejac1993; Richardson et al., Reference Richardson, Peharda, Kennedy, Kennedy and Onofri2004; García-March et al., Reference García-March, García-Carrascosa, Pena Cantero and Wang2007a). However, dense populations have been recently reported from unvegetated bottoms as well (Katsanevakis, Reference Katsanevakis2006; Katsanevakis & Thessalou-Legaki, Reference Katsanevakis and Thessalou-Legaki2009). Pinna nobilis is the largest bivalve in the Mediterranean reaching up to 120 cm, its usual size ranging from 20 to 40 cm (Poutiers, Reference Poutiers, Fischer, Bauchot and Schneider1987; Zavodnik et al., Reference Zavodnik, Hrs-Brenko, Legac, Boudouresque, Avon and Gravez1991), with a life span up to 27 yr (García-March et al., Reference García-March, Surge, Lees and Kersting2011). It is an epibenthic suspension feeder of particular ecological importance, since it is actively involved in benthic–pelagic coupling, contributing to the transport of particulate organic matter of both vegetal and animal origin as revealed by stomach content (Davenport et al., Reference Davenport, Ezgeta-Balic, Peharda, Skejic, Nincevic-Gladan and Matijevic2011), stable isotope (Cabanellas-Reboredo et al., Reference Cabanellas-Reboredo, Deudero and Blanco2009a) and fatty acid analyses (Najdek et al., Reference Najdek, Blazina, Ezgeta-Balic and Peharda2013). Moreover, it provides habitat for numerous species by adding physical structure through its shell, thus enhancing local biodiversity (Cosentino & Giacobbe, Reference Cosentino and Giacobbe2008)—more than 145 species have been reported as epibionts of P. nobilis (Rabaoui et al., Reference Rabaoui, Tlig-Zouari, Cosentino and BenHassine2009). Accordingly, the fan mussel can be assigned among ecosystem engineering organisms in the marine benthic environment since it fulfils the criteria introduced by Jones et al. (Reference Jones, Lawton and Shachak1994).
The fan mussel has been collected and exploited in the Mediterranean since prehistoric times. In antiquity it was a gastronomic delicacy, cooked with oil, wine and honey (Voultsiadou et al., Reference Voultsiadou, Koutsoubas and Achparaki2010). It was harvested for its byssus from which ‘sea silk’, an extremely fine fabric, was produced, considered among the most luxurious of textiles (Carannante, Reference Carranante2010; Voultsiadou et al., Reference Voultsiadou, Koutsoubas and Achparaki2010); nowadays one last atelier can be found in Sicily. Fan mussels have also been utilized as utensils and ornaments as late as the Bronze Age, especially on the Aegean and the Adriatic coasts of Italy (Karali, Reference Karali1986, Reference Karali1999; Carannante, Reference Carranante2010). In recent times, P. nobilis was exploited for food or for decorative items: manufactures, nacre buttons and the brown–red pearls that are produced by the species, although of minor commercial value (Gauthier et al., Reference Gauthier, Caseiro and Lasnier1994). It is harvested by diving or using dredges and bottom nets (Poutiers, Reference Poutiers, Fischer, Bauchot and Schneider1987), while special tools have been developed in some islands of the Aegean Sea (Zachariou-Mamaliga, Reference Zachariou-Mamaliga1986). Fisheries, combined with habitat degradation have caused a severe decline of the P. nobilis population in the Mediterranean (Katsanevakis & Thessalou-Legaki, Reference Katsanevakis and Thessalou-Legaki2009) and the species has been recognized as endangered: since 1992 P. nobilis has been listed under protection (92/43/EC; PD/227/2003) and its harvesting is prohibited in all European countries.
Pinna nobilis is a well studied organism; several aspects of the species biology have been studied such as reproduction (de Gaulejac et al., Reference de Gaulejac, Henry and Vicente1995a, Reference de Gaulejac, Henry and Vicenteb; Richardson et al., Reference Richardson, Peharda, Kennedy, Kennedy and Onofri2004; Peharda & Vilibic, Reference Peharda and Vilibic2008; Cabanellas-Reboredo et al., Reference Cabanellas-Reboredo, Deudero, Alos, Valencia, March, Hendriks and Alvarez2009b), feeding (Cabanellas-Reboredo et al., Reference Cabanellas-Reboredo, Deudero and Blanco2009a; Davenport et al., Reference Davenport, Ezgeta-Balic, Peharda, Skejic, Nincevic-Gladan and Matijevic2011; Najdek et al., Reference Najdek, Blazina, Ezgeta-Balic and Peharda2013), biometry (García-March & Ferrer, Reference García-March and Ferrer1995; García-March et al., Reference García-March, Carcía-Carrascosa and Peña2002) and population ecology (Zavodnik, Reference Zavodnik1967; de Gaulejac & Vicente, Reference de Gaulejac and Vicente1990; Butler et al., Reference Butler, Vicente and de Gaulejac1993; Richardson et al., Reference Richardson, Kennedy, Duarte, Kennedy and Proud1999; Siletic & Peharda, Reference Siletic and Peharda2003; Centoducati et al., Reference Centoducati, Tarsitano, Bottalico, Marvulli Lai and Crescenzo2006; García-March et al., Reference García-March, Rafael and Vicente2006, Reference García-March, García-Carrascosa, Pena Cantero and Wang2007a, Reference García-March, Pérez-Rojas and García-Carrascosab; Rabaoui et al., Reference Rabaoui, Tlig-Zouari, Cosentino and BenHassine2008, Reference Rabaoui, Tlig-Zouari, Katsanevakis and BenHassine2010; Coppa et al., Reference Coppa, de Lucia, Magni, Domenici, Antognarelli, Satta and Cuco2013). The bulk of information, however, has been derived from the Adriatic and the central and western Mediterranean. Relevant data from the eastern Mediterranean are scarce. The existing information is limited to baseline information concerning its genetic and population structure, spatially restricted to two localities of the north Aegean (Galinou-Mitsoudi et al., Reference Galinou-Mitsoudi, Vlahavas and Papoutsi2006; Katsares et al., Reference Katsares, Tsiora, Galinou-Mitsoudi and Imsiridou2008). A comprehensive study of the species density has been conducted within a small marine lake in Korinthiakos Gulf (Katsanevakis Reference Katsanevakis2006, Reference Katsanevakis2007). Recently, the spatial distribution of the species has been estimated in a closed bay on the north-west of Crete (Katsanevakis & Thessalou-Legaki, Reference Katsanevakis and Thessalou-Legaki2009), constituting the only available reference for the species from the southern part of the Aegean Sea. In the latter area, and specifically the Dodecanese, the fan mussel has been traditionally exploited since the middle of the 19th century (Zachariou-Mamaliga, Reference Zachariou-Mamaliga1986). Even nowadays, despite official fishing prohibitions, it is intensely harvested sometimes in parallel with sponges and ascidians, and it is still consumed as fresh or marinated product.
Considering all the above, the present paper aims to assess the current status of P. nobilis populations in the Dodecanese complex (south Aegean Sea) by implementing non-destructive sampling techniques, in order to gather information on density and biometry at spatio-temporal scales. Moreover, an attempt is made to describe the situation of fan-mussel harvesting over the years in this area by compiling data from literature and stakeholder interviews.
MATERIALS AND METHODS
The study was carried out in the Dodecanese island complex in the south Aegean Sea, a typical oligotrophic area characterized by warm (16–27oC) and saline (around 39 psu) waters (Voultsiadou et al., Reference Voultsiadou, Vafidis and Antoniadou2008). Two stations were randomly selected on each of the six islands (Figure 1) surveyed in summer (2005 or 2007) to assess abundance and size structure of P. nobilis populations. Samplings included diving in two depth zones: (1) 0.5–15 m, and (2) 15–30 m, by one scientist and one sponge fisherman using the surface air supply method. In Astypalaia stations six temporal samplings were also accomplished on fixed transects to assess temporal trends of population parameters (March 2004, October 2004, December 2004, June 2005, September 2005 and June 2006). Population density was directly estimated using strip transect sampling (Garcia-March et al., 2007a). Thus, two replicate transects 2 × 200 m—covering 400 m2 each—were surveyed per depth zone at each station, and all living P. nobilis individuals were counted. Pinna nobilis shells were measured in situ for minimum width (w) and unburied length (uL)—to 0.5 cm precision—to estimate maximum antero-posterior shell length (tL). For this purpose we used the empirical equation of Garcia-March & Ferrer (1995) adopted by various authors (Garcia-March et al., 2002, 2007a; Siletic & Peharda, Reference Siletic and Peharda2003; Addis et al., Reference Addis, Secci, Brundu, Manunza, Corrias and Cau2009). At all stations the sea bottom consisted of sandy detritic sediments on which Caulerpa racemosa carpets, small rocky reefs, and rather sparse Posidonia oceanica patches were interspersed at the shallower depth zone; a uniform dense Posidonia meadow occurred at the lower depth zone, where rocky reefs were only occasionally observed.

Fig. 1. Assessment of Pinna nobilis in the marine area of the Dodecanese. Two sampling stations (No. 1 at a depth between 0.5 and 15 m and No. 2 at a depth between 15 and 30 m) were randomly located on each of the six island surveyed (As, Astypalaia; Pa, Patmos; Ar, Arkoi; Ps, Pserimos; Le, Leros; Sy, Symi).
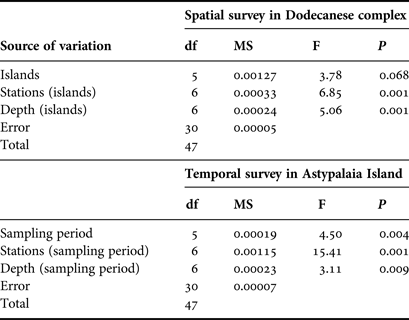
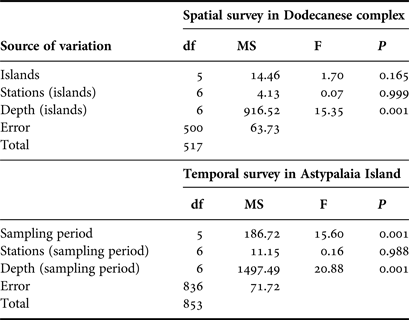
Nested analysis of variance was used to examine differences in population density and shell length of P. nobilis populations between islands, stations and depth zones using the general linear model (Underwood, Reference Underwood1997) (GLM ANOVA, island and depth zone treated as fixed factors, station treated as random factor). Prior to the analyses, data were tested for normality with the Anderson–Darling test, while the homogeneity of variances was tested with Cohran's test and, when necessary, data were log-transformed. The Bonferroni test was used for post hoc comparisons. The same model of ANOVA was used to test for temporal differences in the studied P. nobilis population from Astypalaia (depth zone treated as fixed and stations as random factor). ANOVAs were performed using the SPSS software package (IBM SPSS statistics v.19).
Size–frequency distributions were constructed per 5 cm classes using tL data (García-March et al., Reference García-March, García-Carrascosa, Pena Cantero and Wang2007a). The modal length was identified applying the NORMSEP analysis using the FISAT software package.
RESULTS
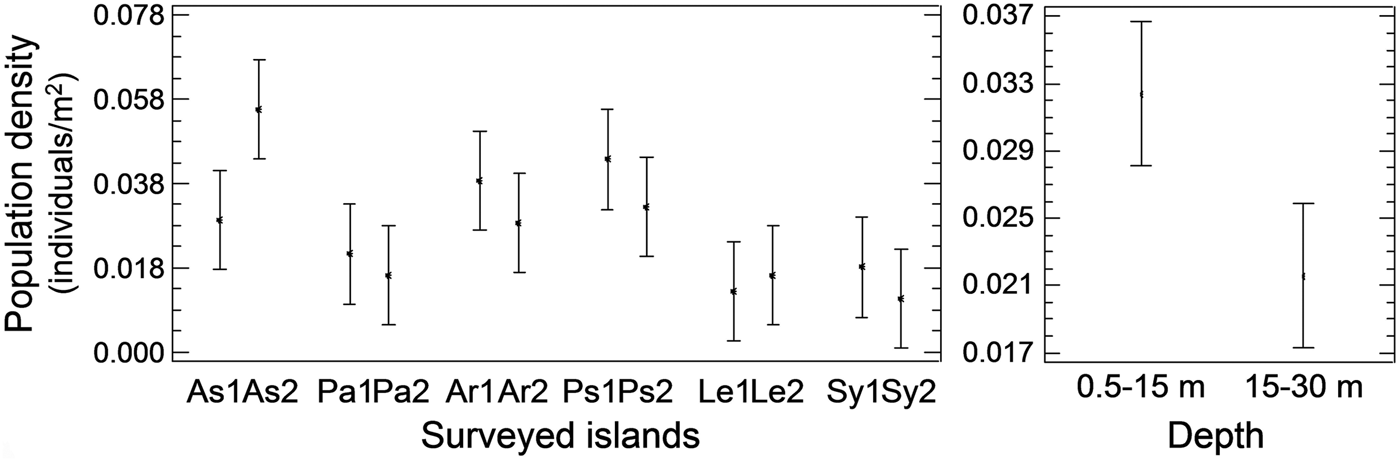
Pinna nobilis was recorded at all surveyed stations, settled either in sandy detritic sediments or Posidonia oceanica meadows. Its population density ranged from 0.005 to 0.073 individuals/m2 with an overall mean (x ± SD) of 0.027 ± 0.015 individuals/m2. Mean density showed significant variation among sampling stations and depth zones, whereas the differences among the surveyed islands were non-significant (ANOVA results, Table 1). Increased density was recorded in the shallower depth zone and in three stations located in Astypalaia (As2), Arkoi (Ar1) and Pserimos (Ps1) Islands (Figure 2). In Astypalaia Island P. nobilis density ranged in time from 0.0775 individuals/m2 (March 2004) to 0.0175 individuals/m2 (September 2005), with an overall mean of 0.044 ± 0.016 individuals/m2 (data pooled over stations and depth zones). Significant differences were detected among sampling periods, stations, and depth zones (ANOVA results, Table 1). Increased density was recorded during the cold period of the year, in As2 and in the shallower depth zone (Figure 3).

Fig. 2. Spatial variability of Pinna nobilis population density at the surveyed stations and depths of the Dodecanese (left graph, data pooled over depth zones per sampling station; right graph, data pooled over stations per depth zone; As, Astypalaia; Pa, Patmos; Ar, Arkoi; Ps, Pserimos; Le, Leros; Sy, Symi; 1, first station, 0.5–15 m depth; 2, second station, 15–30 m depth).

Fig. 3. Temporal variability of Pinna nobilis population density in the surveyed stations and depths of Astypalaia Island (left graph, data pooled over stations and depth zones, per sampling period; middle graph, data pooled over depth zones and sampling periods, per sampling station; right graph, data pooled over stations and sampling periods, per depth zone).
Table 1. ANOVA results of spatio-temporal effects on the surveyed P. nobilis population density (N = 48 for the spatial case and N = 48 for the temporal case).

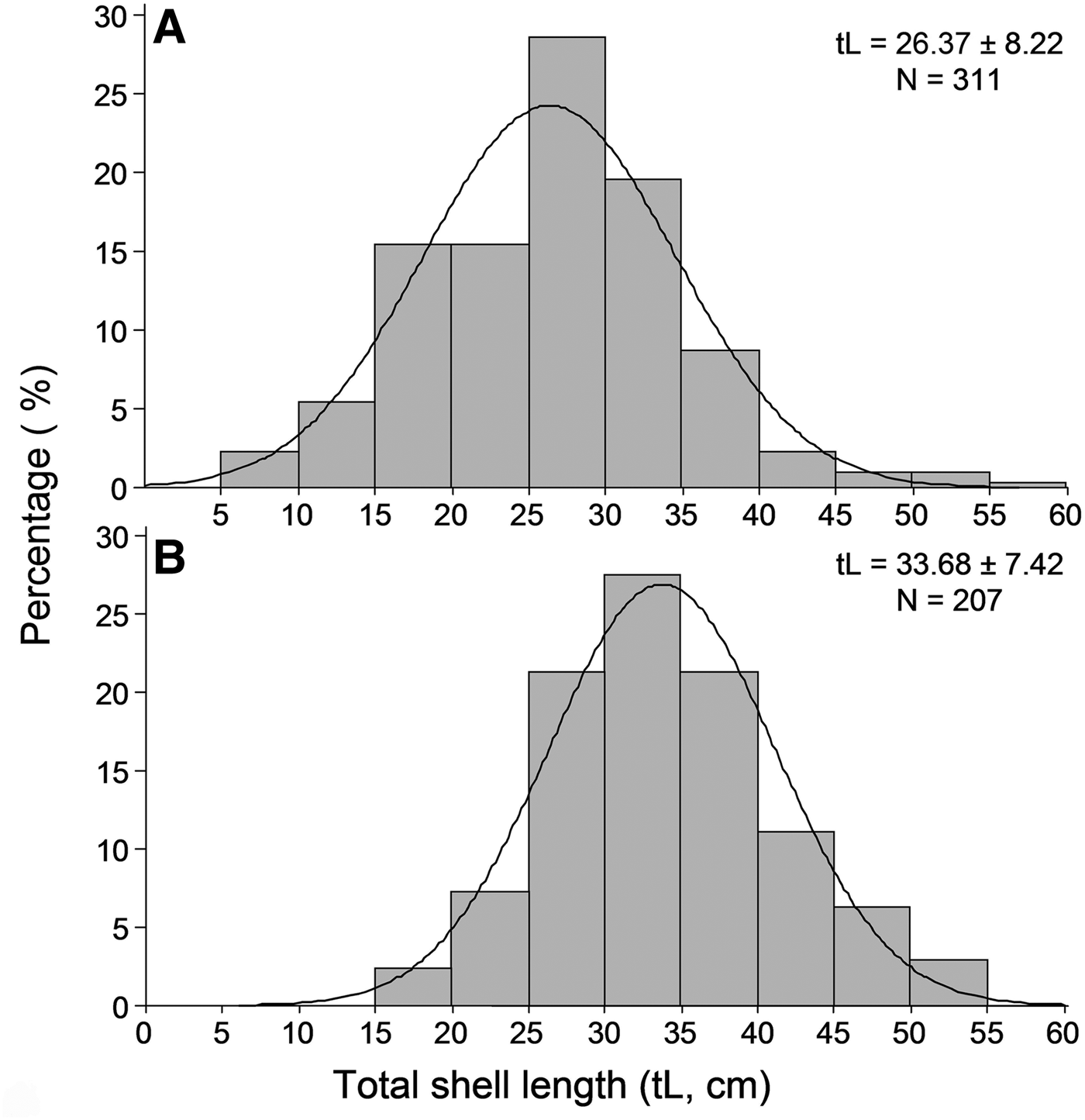
Overall 518 individuals were measured to describe the size structure of the studied population at spatial scales; 311 at the shallower depth zone and 207 individuals in the deeper one. The smallest fan mussel measured 7.5 cm in length and the largest 56.5 cm; the mean length of the studied population was 29.3 ±8.7 cm (±SD). Mean length showed significant variation between depth zones, with increased values in the deeper one (Figure 4), whereas the relevant differences, considering either the surveyed islands or the sampling stations, were non-significant (ANOVA results, Table 2). As only depth had a significant effect on P. nobilis length, size–frequency distributions were constructed for each depth zone separately (data were pooled over stations and islands). The studied population was normally distributed with modes at 26 and 34 cm in the shallow and deeper zones, respectively (Figure 5).

Fig. 4. Spatio-temporal variability of total shell length in the studied Pinna nobilis populations according to the factors for which significant effects were detected (left graph, data pooled over stations and islands of the Dodecanese, per depth zone; middle graph, data pooled over depth zones and sampling stations of Astypalaia Island, per sampling period; right graph, data pooled over stations and sampling periods of Astypalaia Island, per depth zone).

Fig. 5. Length–frequency distribution of the studied Pinna nobilis population in the 0.5–15 m (A) and 15–30 m (B) depth ranges surveyed (data were pooled over stations and islands).
Table 2. ANOVA results of spatio-temporal effects on maximum shell length of the surveyed P. nobilis population (N = 518 for the spatial case and N = 854 for the temporal case).

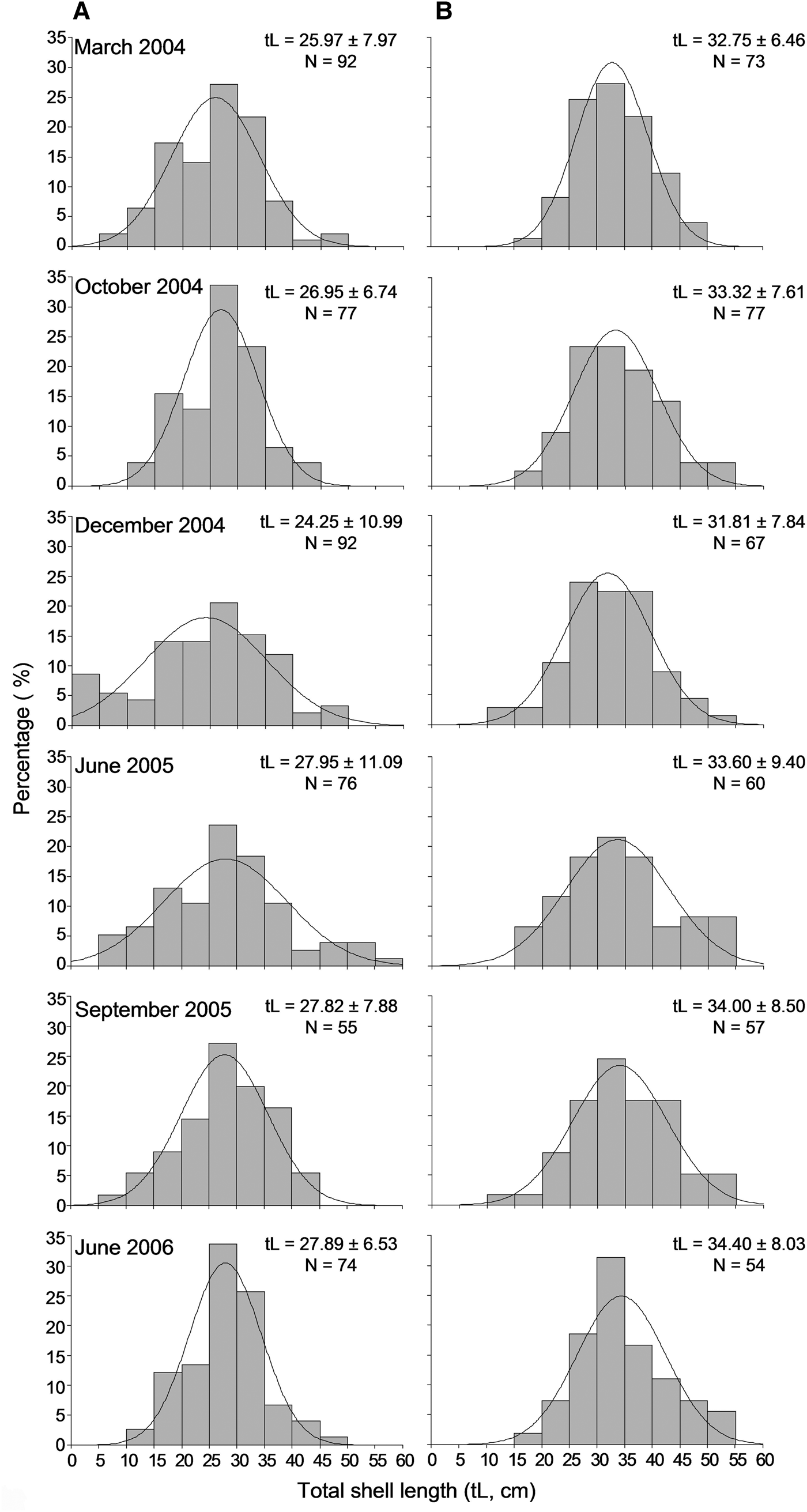
A sample of 854 individuals was measured to analyse the size structure of the P. nobilis population in Astypalaia Island at temporal scales; 466 at the shallower depth zone and 388 individuals in the deeper one. The smallest fan mussel measured 4.0 cm in length and the largest 57 cm; the mean length of the studied population was 29.6 ±9.1 cm (×SD). Mean length showed significant variation between depth zones and temporal samplings, whereas the differences between sampling stations were non-significant (ANOVA results, Table 2). Smaller individuals were detected in the shallower depth zone and in December (Figure 4). Accordingly, size–frequency distributions of P. nobilis length were constructed for each depth zone and temporal sampling separately (data were pooled over stations). The studied population was normally distributed with modes temporally ranging from 24 to 30 cm and from 28 to 36 cm in the shallow and deeper zones, respectively (Figure 6). In December 2004 distribution shifted leftwards due to the increased number of small-sized individuals.

Fig. 6. Length–frequency distribution of the studied Pinna nobilis population per sampling period in the 0.5–15 m (A) and 15–30 m (B) depth ranges surveyed (data were pooled over stations).
DISCUSSION
In the Dodecanese the density of P. nobilis is rather low in comparison with other Aegean sites surveyed, where it can reach 0.2 or even 1.3 individuals/m2 in the south (Katsanevakis & Thessalou-Legaki, Reference Katsanevakis and Thessalou-Legaki2009) and north sectors (Galinou-Mitsoudi et al., Reference Galinou-Mitsoudi, Vlahavas and Papoutsi2006), respectively. Comparatively dense populations were only locally detected in very sheltered bays. This result may be linked with the oligotrophic status of the Dodecanese area (Antoniadou & Vafidis, Reference Antoniadou and Vafidis2011) creating constraints on fan mussel feeding. Pinna nobilis is a large-size suspension feeder with high energetic requirements. Detailed ecophysiological or bioenergetics studies have not so far been conducted for the species, but a mean filtration rate of 5.99 l h−1 g−1 has been estimated (Riva, 2012). Although it consumes both phyto- and zooplankton, detritus makes up the bulk of ingested material (Cabanelas-Reboredo et al., 2009a; Davenport et al., Reference Davenport, Ezgeta-Balic, Peharda, Skejic, Nincevic-Gladan and Matijevic2011); accordingly, the species can thrive in areas with increased organic input, such as coastal salt marshes, estuaries, lagoons and protected inlets (García-March et al., Reference García-March, García-Carrascosa, Pena Cantero and Wang2007a; Addis et al., Reference Addis, Secci, Brundu, Manunza, Corrias and Cau2009).
Fishing pressure is another factor that could have severely affected P. nobilis density in the studied area. In the Aegean Sea the fan mussel fishery goes back to the middle of the 19th century when small-scale fishermen developed specific gears to collect pinnids (Zachariou-Mamaliga, Reference Zachariou-Mamaliga1986). Apart from the targeted fan mussel fishery, P. nobilis harvesting developed in parallel with sponge fishery practised by skilful divers from Kalymnos and Symi Islands in the south Aegean Sea (Voultsiadou et al., Reference Voultsiadou, Dailianis, Antoniadou, Vafidis, Dounas and Chintiroglou2011). Sponge fishermen and, after the collapse of sponge fishing grounds, shellfish fishermen targeting the ascidian Microcosmus sabatieri (Vafidis et al., Reference Vafidis, Antoniadou and Chintiroglou2008), started to collect P. nobilis as a by-catch throughout the Aegean Sea. This harvesting was most intense in the southern part, where the main fishing grounds of the ascidian extend (Antoniadou & Vafidis, Reference Antoniadou and Vafidis2008). Nowadays there are about 45 small-scale fishery vessels harvesting and processing P. nobilis, despite the official restrictions, all harboured at Symi and Kalymnos Islands. There are no official data on P. nobilis landings, but an annual production of 1.5 t (de-shelled wet weight) is estimated according to the Sponge-Fishermen Association of Kalymnos. All catches are sold as fresh product in the local market, especially in Symi Island where the species is also sold as marinated product. Despite fishing prohibitions, P. nobilis is served quite often in many seafood restaurants throughout Greece, in the Aegean islands in particular (Katsanevakis et al., Reference Katsanevakis, Poursanidis, Issaris, Panou, Petza, Vassilopoulou, Chaldaiou and Sini2011).
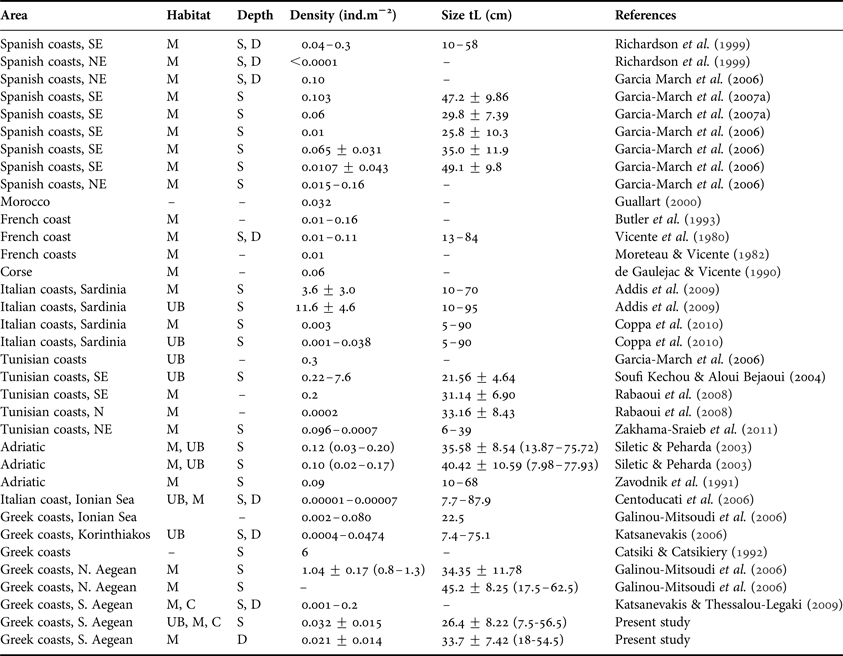
It is fairly difficult to evaluate the P. nobilis population status in the Mediterranean over the years, since published data offer only fragmentary information, as has been stressed by several authors (see García-March et al., Reference García-March, García-Carrascosa, Pena Cantero and Wang2007a). The density of fan mussel populations is among the most studied parameters (see Table 3, updating existing information over the Mediterranean). In general P. nobilis forms sparse (about 0.01 individuals/m2) metapopulations with dense localized patches (about 0.16 individuals/m2) scattered in-between (Butler et al., Reference Butler, Vicente and de Gaulejac1993; García-March et al., Reference García-March, García-Carrascosa, Pena Cantero and Wang2007a). This pattern, although not fully understood, has been attributed to the occurrence of appropriate environmental conditions, such as hydrodynamics and food, instead of gregarious settlement (García-March et al., Reference García-March, Rafael and Vicente2006, Reference García-March, García-Carrascosa, Pena Cantero and Wang2007a, Reference García-March, Pérez-Rojas and García-Carrascosab; Coppa et al., Reference Coppa, de Lucia, Magni, Domenici, Antognarelli, Satta and Cuco2013).
Table 3. Densities and maximum antero-posterior shell length (tL) of Pinna nobilis reported from various Mediterranean areas considering habitat (M, meadows; UB, unvegetated bottoms; C, algal carpets) and depth (S, down to 15 m depth–not exceeding 5 m in most cases; D, below 15–35 m depth).

A significant decrease in P. nobilis density with depth, i.e. below 15 m, was found in the present study. Depth is a well documented factor influencing the species' density (Siletic & Peharda, Reference Siletic and Peharda2003; García-March et al., Reference García-March, García-Carrascosa, Pena Cantero and Wang2007a). A positive relation with depth has been reported to occur from the surface down to the isobaths of 10–15 m, which seems to be the favoured depth zone for the species distribution (Zavodnik, Reference Zavodnik1967; Katsanevakis & Thessalou-Legaki, Reference Katsanevakis and Thessalou-Legaki2009). Small-scale environmental regimes, hydrodynamics in particular, have been proposed as the shaping factor of P. nobilis depth distribution (García-March et al., Reference García-March, García-Carrascosa, Pena Cantero and Wang2007a, Reference García-March, Pérez-Rojas and García-Carrascosab; Coppa et al., Reference Coppa, de Lucia, Magni, Domenici, Antognarelli, Satta and Cuco2013). The occurrence of different habitat types may be another factor involved. In shallow depths P. nobilis has usually been reported on unvegetated sediments or Caulerpa racemosa carpets, or seagrass meadows, whereas deeper the species is found only within the latter habitat type (Richardson et al., Reference Richardson, Kennedy, Duarte, Kennedy and Proud1999; Siletic & Peharda, Reference Siletic and Peharda2003; Galinou-Mitsoudi et al., Reference Galinou-Mitsoudi, Vlahavas and Papoutsi2006; Katsanevakis, Reference Katsanevakis2006). Higher population densities have been reported from the former habitats, possibly indicating enhanced recruitment success or lower natural mortality rates (Katsanevakis & Thessalou-Legaki, Reference Katsanevakis and Thessalou-Legaki2009). In the Mediterranean, seagrass meadows usually extend from the mean sea level down to 40 m, following species substitution with depth (Duarte, Reference Duarte1991); Cymodocea nodosa is usually limited to shallow depths (15–20 m), whereas P. oceanica is found in deeper waters (20–35 m). These two species have different morphological architecture, thus providing habitats of different complexity at different depths. Moreover, considering the same seagrass species, the morphology of a meadow is differentiated with depth; in the case of P. oceanic, shoot density, leaf length, and dead tips are shorter in the shallow stands, in contrast with the number of leaves per shoot and leaf blade width, which increase at the deep stands of the meadow (Dalla Via et al., Reference Dalla Via, Sturmbauer, Schonweger, Sotz, Mathekowitsch, Stifter and Rieger1998), the latter becoming homogeneous below 15 m depth (Fornes et al., Reference Fornes, Basterretxea, Orfila, Jordi, Alvarez and Tintore2006). In the study area a dense, well-developed P. oceanica meadow prevailed below 15 m, whereas in shallower depths mixed sediments occurred (boulders, sand, biogenic detritic sediments, C. racemosa carpet, rather small, sparse P. oceanica patches). Such habitat-related differences could have a significant effect on P. nobilis recruitment and survival (Katsanevakis & Thessalou-Legaki, Reference Katsanevakis and Thessalou-Legaki2009), while possible sampling artefacts cannot be excluded, as detection ability differs between habitats, especially when considering small fan mussels. Therefore, further research is needed to explain the increased abundance of P. nobilis in the shallower depth zone.
Fan mussel density varied in time, with increased values during the cold period of the year. Such a pattern can be related either to the reproduction of the species or to differences in recruitment success. Very few studies have examined P. nobilis reproduction and recruitment (de Gaulejac & Vicente, Reference de Gaulejac and Vicente1990; Butler et al., Reference Butler, Vicente and de Gaulejac1993; de Gaulejac et al., Reference de Gaulejac, Henry and Vicente1995a, Reference de Gaulejac, Henry and Vicenteb; Richardson et al., Reference Richardson, Peharda, Kennedy, Kennedy and Onofri2004; Cabanellas-Reboredo et al., Reference Cabanellas-Reboredo, Deudero, Alos, Valencia, March, Hendriks and Alvarez2009b). Although comprehensive histological examination of the species gonads is scarce (de Gaulejac et al., Reference de Gaulejac, Henry and Vicente1995a, Reference de Gaulejac, Henry and Vicenteb), spawning has been estimated to take place between March and September, and the released larvae settle from late summer to autumn (Richardson et al., Reference Richardson, Peharda, Kennedy, Kennedy and Onofri2004; Cabanellas-Reboredo et al., Reference Cabanellas-Reboredo, Deudero, Alos, Valencia, March, Hendriks and Alvarez2009b). Reproduction in bivalves shows high inter-annual variability and is strongly influenced by seawater temperature, with a spring advancement of spawning and reduced output in warmer marine regions (Philippart et al., Reference Philippart, van Aken, Beukema, Bos, Cadee and Dekker2003); therefore, recruitment can be expected to occur in late spring–early summer in the Dodecanese. Apart from temporal differences in recruitment success that could have shaped adult population structure, fishing pressure is another factor involved. Sponge fishermen target P. nobilis in specific periods of the year; their fishery season extends from late April to late October, and in the winter they cease diving. The observed decline in density from 2004 to 2005 and 2006 possibly indicates overfishing, although relevant data have been collected in different seasons of the year. However, to ascertain the above trend additional data are required covering extensive time periods (i.e. >10 yr), as P. nobilis is a slow-growing, long-lived species.
At both spatial and temporal scales, the studied P. nobilis populations consisted of much larger individuals in the deeper zone. Such a pattern conforms to previous reports from western Mediterranean populations, suggesting a size-segregated pattern with depth, being explained by hydrodynamic differences. The more intense water movement in shallow and exposed habitats results quite often in the detachment of large-shelled individuals (García-March et al., Reference García-March, García-Carrascosa, Pena Cantero and Wang2007a; Reference García-March, Pérez-Rojas and García-Carrascosab), whereas in deeper waters the dense, homogeneous P. oceanica meadows (Fornes et al., Reference Fornes, Basterretxea, Orfila, Jordi, Alvarez and Tintore2006) offer additional protection to large fan mussels. However, as shallow fan mussel stocks have a different growth rate to the deeper ones (García-March et al., Reference García-March, Pérez-Rojas and García-Carrascosa2007b), other factors, abiotic or biotic, are probably also involved, such as food availability and intrinsic genetic structure; for example, increased intrapopulation haplotypic diversity has been reported within Aegean populations of the species (Katsares et al., Reference Katsares, Tsiora, Galinou-Mitsoudi and Imsiridou2008). Several authors suggest different recruitment success in different habitats, with C. racemosa carpets favouring settlement or survival of larvae (Katsanevakis & Thessalou-Legaki, Reference Katsanevakis and Thessalou-Legaki2009); this habitat type is common in the surveyed shallow zones, and may have contributed to the increased presence of small-sized individuals. Another possible explanation could be the preferential harvesting of large-sized P. nobilis by amateurs and fishermen, who continue to poach the species despite relevant prohibitions in EU and national legislation, a phenomenon which seems to be widespread over Greek territory (Katsanevakis et al., Reference Katsanevakis, Poursanidis, Issaris, Panou, Petza, Vassilopoulou, Chaldaiou and Sini2011). In this case as well, the lower detectability of small-sized individuals in deeper waters due to the dense cover of P. oceanica shoots may also be involved.
The significantly smaller size observed in winter due to the increased presence of small-sized juveniles was observed in both depth zones—though it was more pronounced in the shallow one—and is probably related to the reproductive biology of the species. As previously discussed, spawning in the Dodecanese is expected to occur in late spring to early summer; thus, first recruits may have been detected in the surveyed population during the winter after attaining appropriate size, that is, over 3 cm, so as to be detectable by divers. Based on the data collected within this study and previous researches on the species recruitment (Cabanellas-Reboredo et al., Reference Cabanellas-Reboredo, Deudero, Alos, Valencia, March, Hendriks and Alvarez2009b) and estimated growth rates (Galinou-Mitsoudi et al., Reference Galinou-Mitsoudi, Vlahavas and Papoutsi2006; García-March et al., Reference García-March, García-Carrascosa, Pena Cantero and Wang2007a), the smallest fan mussels detected measuring 4 cm were about 5–6 months old, implying that larval settlement in the Dodecanese occurs in June–July. However, as settlement and recruitment are highly complex procedures depending on local conditions (Cabanellas-Reboredo et al., Reference Cabanellas-Reboredo, Deudero, Alos, Valencia, March, Hendriks and Alvarez2009b), further research is required to assess relevant patterns of P. nobilis populations in the south Aegean.
According to length–frequency distribution analysis, the studied P. nobilis population is composed of small or medium sized individuals when compared to other Mediterranean populations (see Table 3). However, the species manifests different growth rates, as the k coefficient of the von Bertalanffy equation was reported to vary considerably between studied populations (Galinou-Mitsoudi et al., Reference Galinou-Mitsoudi, Vlahavas and Papoutsi2006). Pinna nobilis is a fast growing species in the first 3–5 yr of life, regardless of the study area, but thereafter growth is much slower and highly variable between different stocks or even between groups of individuals inhabiting different depths at the same habitat (Richardson et al., Reference Richardson, Kennedy, Duarte, Kennedy and Proud1999, Reference Richardson, Peharda, Kennedy, Kennedy and Onofri2004; García-March et al., Reference García-March, García-Carrascosa, Pena Cantero and Wang2007a). The above characteristics have important implications for the species conservation, since the largest and oldest bivalves are more fecund and consequently of conservation priority against poaching (Peharda & Vilibic, Reference Peharda and Vilibic2008).
Summarizing, P. nobilis populations in the Dodecanese follow the general spatial distribution pattern described for the species in other Mediterranean areas, with extended, sparse populations and sporadic presence of dense patches in shallow (<15 m) sheltered areas. A pattern of depth-related density and size segregation, with fewer but larger individuals in deeper waters was observed, while the population became denser in the cold period of the year, during which new recruits enter causing a decline in P. nobilis size. Differences in local environmental conditions, hydrodynamics, and habitat types, are proposed to shape P. nobilis population structures, although factors such as illegal fishing cannot be excluded.
ACKNOWLEDGEMENTS
We are grateful to the Coastal Fishermen Association of Kalymnos, ‘Panagia-Ypapanti’, the Sponge-Fishermen Association of Kalymnos, and the captains and the crew of the sponge fishing vessels ‘Themelis NK168’ and ‘Cap. Spyros NK58’ for their help during samplings.
FINANCIAL SUPPORT
This research was financially supported by the Greek Ministry of Rural Development and Food over the period 2000–2006 (EPAL 2000–2006; Grant number 101050/2).